|
|
Symplegma brakenhielmi
(Michaelsen, 1904), Styelidae Ascidian
|
|
Melissa Nancy Staines 2017
|
|
|
|
Summary | |
Ascidiacea is the sessile class of the sub-phylum Urochordata (phylum Chordata), and come in a large variety of morphologies and range of complexity (Ruppert, et al, 2004). Some of the most complex ascidians are the colonial (compound) species, that function as a superorganism but are controlled by tiny zooids (Ruppert, et al, 2004). Their obscure adult body plan is unlike any other phyla. The tadpole larva is more neurologically complex than the adult, and is the only morphological evidence that relates ascidians to chordates (Ruppert, et al, 2004). Ascidians serve an important ecological role in the benthos; they are food for many small predatory invertebrates (Simkanin, et al, 2013) and they filter the water for food particles (phytoplankton and bacteria), improving the quality of the water (Stabili, et al, 2016).
Symplegma brakenhielmi is a species of invasive colonial ascidian, first described by Michaelsen in 1904. This species belongs to a larger family known as Styelidae (Polyzoinae), where zooids are loosely integrated within a common tunic, but share a sophisticated vascular network (Kott, 2004). Zooids share hemocytes (blood cells) and nutrients through this complex vascular network in order to produce new zooids (Gutierrez and Brown, 2017). Zooids can grow up to 3mm wide and have a variety of colour morphs; green, orange, white, yellow and red (Kott, 2004).
This species owes its widespread distribution to the colony's ability to asexually reproduce and alter between reproductive strategies (Gutierrez and Brown, 2017). S. brakenhielmi can rapidly increase it's colony density over a matter of a few days, using palleal, stolonic or vascular budding to produce zooids (Gutierrez and Brown, 2017). Zooids are also able to recycle biological material through the reabsobtion of older zooids, making it appear as if the colonial was migrating along the substrate (Gutierrez and Brown, 2017). Their lecithotrophic larva do not feed, thus dispersal distance is restricted and leads to low genetic variation between local populations (Dias, et al, 2006).
 S. brakenhielmi show characteristics of both solitary (ancestral) and colonial (derived) ascidian species (Gutierrez and Brown, 2017). Zooids grow asynchronously and have a non-polar orientation, they filter-feed independently of each other, but the zooids still share a common tunic and vascular network and primarily reproduce asexually (Gutierrez and Brown, 2017). This identifies the S. brakenhielmi as a possible transitional species between the solitary Styelidae (Styela) and colonial Styelidae (Botrillinae) (Gutierrez S. brakenhielmi show characteristics of both solitary (ancestral) and colonial (derived) ascidian species (Gutierrez and Brown, 2017). Zooids grow asynchronously and have a non-polar orientation, they filter-feed independently of each other, but the zooids still share a common tunic and vascular network and primarily reproduce asexually (Gutierrez and Brown, 2017). This identifies the S. brakenhielmi as a possible transitional species between the solitary Styelidae (Styela) and colonial Styelidae (Botrillinae) (Gutierrez
and Brown, 2017).
Figure 1: Symplegma brakenhielmi colony attached to coral in
the Mediterranean sea. Image source: Kott, 2004.
S. brakenhielmi thrives in warm-waters; colonies can grow at high densities on man-made objects like pylons, buoys and in aquaculture systems, and the species has been found across the pan-tropic range of the world's oceans (Kott, 2004). The S. brakenhielmi specimens used for this project were collected from a mussel clump at North Stradbroke Island, Queensland, and can be found along most of the coastlines of Australia (Kott, 2004). Colonial ascidians like S. brakenhielmi have recently become a large problem in the mussel aquaculture industry, reducing productivity of the stock (Paetzold, et al, 2012). Successful implements to stop outbreaks are still in development (Paetzold, et al, 2012).
|
|
|
Physical Description | |

Symplegma brakenhielmi (Michaelsen in 1904) is a compound Styelidae ascidian from the Stolidobranchia clade (stolido = folded in greek) (Monniot, et al, 1991). Each mature zooid is only 3mm wide and 3mm tall (figure 2), they are dorso-ventrally flattened and attached to the strata via their their ventral (basal) surface (Kott 2004). It isn't until you look at a zooid under a microscope when you get to see their real beauty.
Figure 2: S. brakenhielmi zooid is approximately 3mm tall (long).
.
.
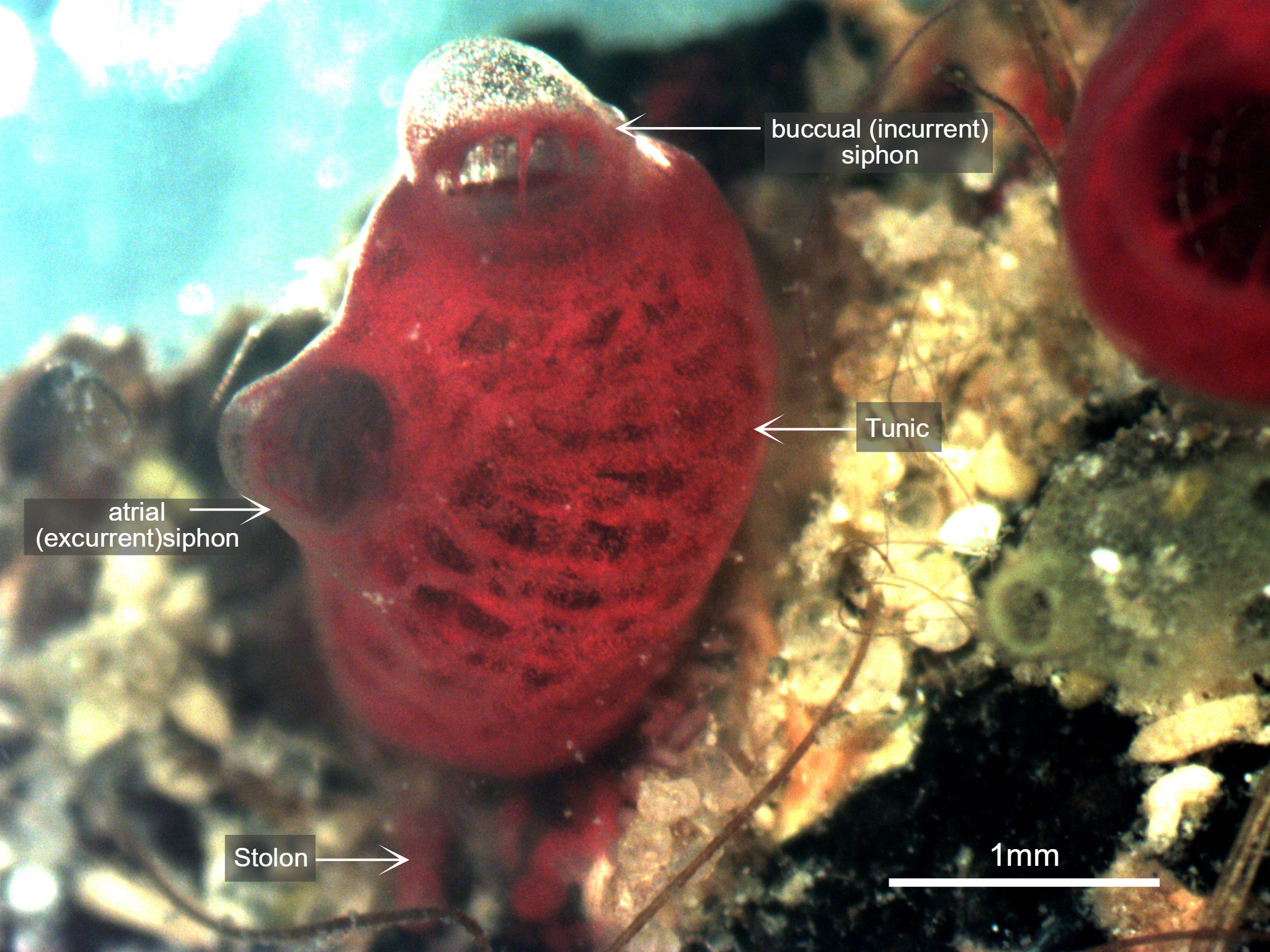
Figure 3: Photograph taken of S. brakenhielmi zooid settled on a mussel shell, clearly showing the buccual (incurrent) siphon with buccual tentacles, and an atrial (excurrent) siphon. The tunic is partially transparent and filled with blood and red pigment cells, and through the tunic, a red branchial basket is visible. Scale: 1mm.
The Tunic
Mature S. brakenhielmi zooids have a partially-translucent tunic (figure 3) that loosely integrates zooids of the colony (Gutierrez & Brown, 2017). The tunic itself is used for structural support and protection, as all ascidians lack CaCo3 skeletons (see Anatomy & Physiology) (Monniot et al. 1991). Because of their size, these zooids do not need to be as robust as their larger solitary relatives (Styela), and the tunic is actually quite soft and contains many proteins, pigments and blood cells (Monniot et al. 1991). In the early stages of their adult development the tunic is secreted by cells of the outer epithelium (Gutierrez & Brown, 2017). Along with structural support and protection, the tunic also helps the zooid to grip to soft or hard stratum, meaning that they are completely sedentary animals (Monniot et al. 1991).
The Siphons
Along the dorsal side of the zooid are two discrete siphons (figure 3); the large buccual (incurrent) siphon and the small atrial (excurrent) siphon (Ruppert, et al, 2004). S. brakenhielmi has a close relative called Symplegma Rubra (Monniot, 1972), and it can be very difficult to tell the two species apart, especially since there distribution overlaps greatly (Rocha and Costa, 2005). The main external differences are characteristics of the siphons; S. brakenhielmi zooids have smaller apertures (openings), and S. rubra has a pigmented ring that extends around both siphons of a zooid (figure 6) (Kott, 2004).
S. rubra has two colour morphs; bright yellow or wine-red, whereas S. bakenhielmi varies between dark green, whitish-yellow, orange-red to pomegranate-red (see figure 4 and 5) (Rocha and Costa, 2005) (Brown and Swalla, 2012) (Kott, 1985).
The Stolons
Stolons are "finger-like" extensions of the internal body wall (mantle), which secrete tunic proteins (tunicin) and contain blood vessels (Monniot et al. 1991). The purpose of thes stolons is to connect zooids together so that they can share hemocytes (stem cells), blood and nutrients, as well as being the source of asexual reproduction (see Asexual Reproduction) (Monniot et al. 1991). The stolons have a single layer of epithelium, which allows a unidirectional flow of blood through the stolons and between the zooids (Brown and Swalla, 2012).
 
Figure 4 (left): S. brakenhielmi colony of loosely integrated zooids (Gutierrez and Brown, 2017). Each zooid has a clear incurrent (large) and excurrent (small) siphon and a partially-translucent tunic, which reveals the bright red-coloured branchial basket (Kott, 1985).
Figure 5 (right): Green morph of S. brakenhielmi colony found near Sydney, NSW Australia. Branchial basket clearly visible through translucent tunic of each zooid and the colony has an irregular growth pattern. Image Source: Australian Museum, 2017.

Figure 6: Photograph of a wild Symplegma rubra colony, clearly showing the two distinguishing features between S. rubra and S. brakenhielmi; a pigmented ring on the anterior side of the animal that surrounds both siphons, and larger apertures of the siphons. Image source: Kott, 2004.
Back to top of page
|
|
|
Ecology | |
Habitat
S. brakenhielmi is a semi-colonial species that has an invasive distribution across the sub-tropic/tropical oceans of the world (see Biogeographic Distribution) and extends along much of the eastern coast of Australia (Kott, 2010). This species can be found as deep as 20m below the water's surface (Kott, 2010), and is found in many harbours attached to man-made objects like bouys and pontoons (Lambert and Lambert, 1998). At North Stradbroke Island, this species is commonly found settled on shipwrecks, under rocks (cryptic), living on mussel clumps and even on the mudflats with Zostera sp. of seagrass (Kott, 2010).
Biofouling
S. brakenhielmi is a biofouling species that inhabits multiple habitats within the marine environment (Kott, 2004). The sample of the species looked at in this project was found on the shell of mussel clumps from Stradbroke Island (figure 7), which normally for a soft-bodied ascidian would not be the safest location to start a colony. However due to their small size, epifaunal ecology and regenerating abilities, this species has become specially adapted for biofouling on poor quality
substrates and invading new waters (Kott, 2004). 
S. brakenhielmi colonies are most commonly
found in harbours, where they can settle on man-made objects (Çinar et al, 2006).
A study by Rosa et al (2013) showed that biofouling colonial ascidians are a potential
threat to the aquaculture industry and could have
a severe ecological impact by introducing toxic
algal blooms into a system. They also compete with aquaculture species for settlement space and food in the water column, devastating mussel and oyster stock (Kang, et al, 2011).
Figure 7: Mussel shell with biofouling S. brakenhielmi attached.
Invasive biofouling ascidians such as this species could also cause some economic stress, as there is a lot of maintenance required to stop the fouling of ship hulls and intake water pipes (Çinar et al, 2006).
Bio-filtration
Ascidians are extremely efficient at filter feeding and can filter particles out of the water between 0.6–100 µm (Rosa et al, 2013). Their filtration rates are even higher than that of bivalves living at the same temperature (Rosa et al, 2013). Colonial ascidians species related to S. brakenhielmi have been shown to act as a local ecosystem biofilter, and increase water quality by filtering out harmful bacteria - bio-filtration could be used in the aquaculture industry to reduce the instances of bacterial disease (Stabili, et al, 2016). Similarly, colonial ascidians like S. brakenhielmi can extract heavy metals and toxic elements from the water as they filter-feed, and become bio-indicators of water quality in their local area (Monniot et al. 1991).
 Predators Predators
Adult colonial ascidians have many natural predators such as crabs and shrimp (decapoda), seastars (asteroidea), nudibranchs (gastropoda), small fish and free swimming flatworm (platyhelminthes) (figure 8) (Simkanin, et al, 2013). Whilst larval ascidians are predated on by most suspension feeding organisms (Olson and McPherson, 1987).
To reduce their risk of being eaten, Symplegma species produce secondary metabolites and inorganic acids to become unpalatable or even toxic (see Chemical
Figure 8: A free-swimming flatworm predating on a semi-colonial Defences in Anatomy and Physiology) (Pisut and
ascidian with its branched pharynx. Image source: http://www.kudalaut.eu/ Pawlik, 2002).
en/dph/752/Photos-Sale/Flatworm
Back to top of page
|
|
|
Life History and Behaviour |
Life Cycle | |
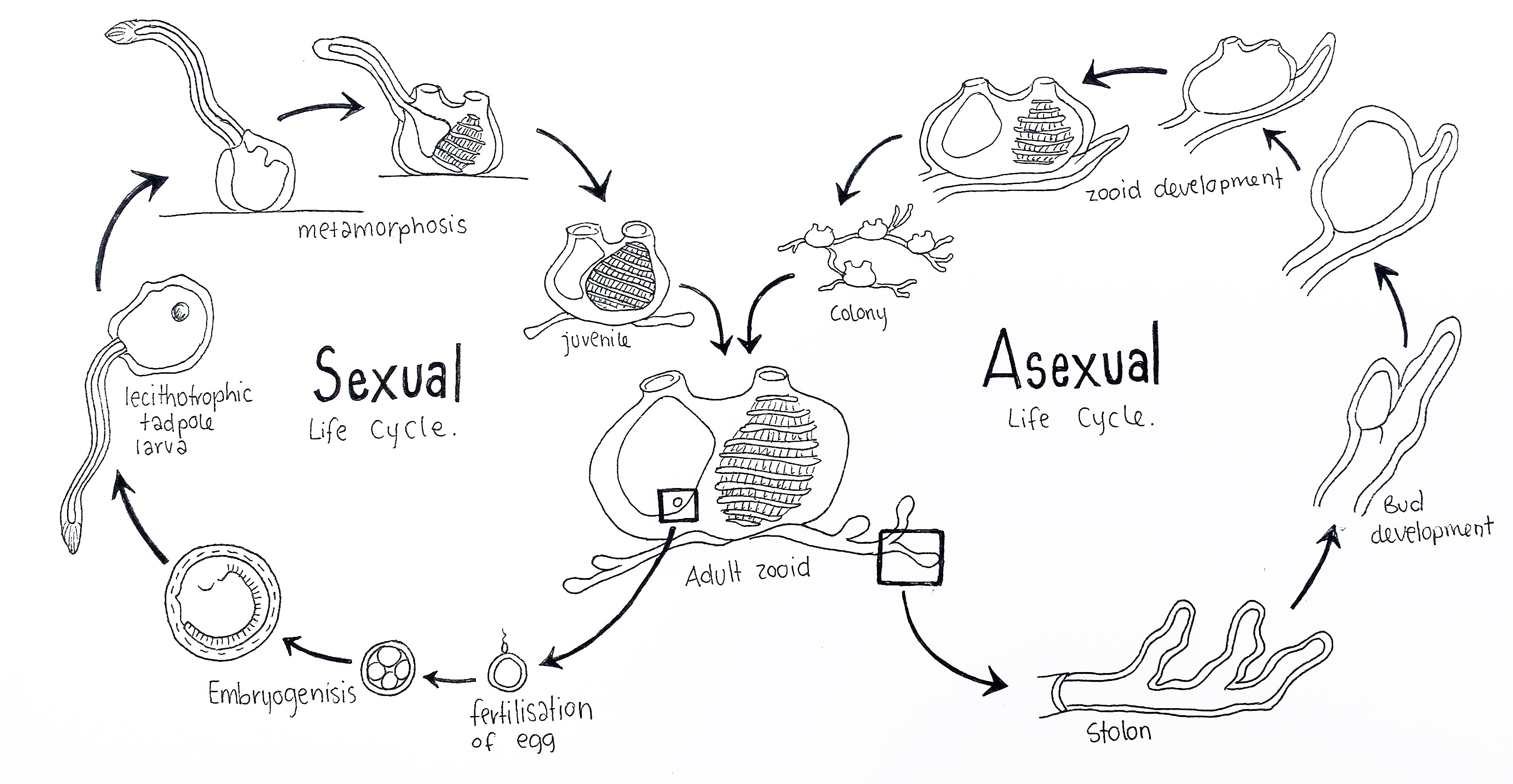 Figure 9: Schematic drawing of the colonial ascidian S. brakenhielmi's life cycles; The sexual life cycle (left) begins with the fertilisation of the egg inside the atrium of an adult blastozooid (benthic life stage). After fertilisation follows blastrulation, gastrulation and embryogenisis, which occurs inside the atrium (brooding). A lecithotrophic larva hatches and swims into the water column (pelagic life stage) and finds a vacant surface to settle on. Next follows metamorphosis of the larva, where the post-anal tail, sense vessicle, notochord and nerve cord are degraded and siphons grow on the dorsal side of the animal. The pharynx metamorphose into a larger filter feeding basket, the juvenile then begins filtration of water and grows into an adult zooid (Swalla, 2006). The Asexual life cycle (right) is variable, as adult zooids can undego pallial, vascular or stolonic budding. Here the stolon forms a bud at an interval, which grows into a juvenile zooid from the supply of hemocytes in the blood of vessels in the stolon. Multiple zooids form a larger colony which are connected by stolonic tissue (Brown and Swalla, 2012). Adapted from Swalla, 2006.
Figure 9: Schematic drawing of the colonial ascidian S. brakenhielmi's life cycles; The sexual life cycle (left) begins with the fertilisation of the egg inside the atrium of an adult blastozooid (benthic life stage). After fertilisation follows blastrulation, gastrulation and embryogenisis, which occurs inside the atrium (brooding). A lecithotrophic larva hatches and swims into the water column (pelagic life stage) and finds a vacant surface to settle on. Next follows metamorphosis of the larva, where the post-anal tail, sense vessicle, notochord and nerve cord are degraded and siphons grow on the dorsal side of the animal. The pharynx metamorphose into a larger filter feeding basket, the juvenile then begins filtration of water and grows into an adult zooid (Swalla, 2006). The Asexual life cycle (right) is variable, as adult zooids can undego pallial, vascular or stolonic budding. Here the stolon forms a bud at an interval, which grows into a juvenile zooid from the supply of hemocytes in the blood of vessels in the stolon. Multiple zooids form a larger colony which are connected by stolonic tissue (Brown and Swalla, 2012). Adapted from Swalla, 2006.
S. brakenhielmi like many colonial ascidians, has a biphasic sexually reproductive life cycle, and a benthic asexually reproductive lifestyle (figure 9) (Ruppert, et al, 2004). Within the colony, there is a differential in reproductive roles between zooids; asexually-reproducing zooids (oozooids) extend on the periphery of the colony, whilst sexually-reproducing zooids (blastozooids) remain in the centre of the colony (Sugimoto and Nakauchi, 1974). When the colony is small, it's priority is to grow larger very quickly using asexual reproduction (Gutierrez and Brown, 2017).
|
|
|
Asexual Reproduction | |
S. brakenhielmi zooid's primary form of reproduction is asexual budding (Gutierrez and Brown, 2017). Within the phylum Ascidiacea there are twelve different types of asexual reproduction (Monniot et al. 1991), but the Symplegma genus has only three known ways for creating new zooids (Gutierrez and Brown, 2017).
1. Palleal budding
This is when buds form from lateral/side walls of the epidermis from an adult (parent) zooid, and develop early adult features whilst inside the tunic (figure 11, top left) (Gutierrez and Brown, 2017).
2. Stolonic budding
This form is where new buds grow from the connective stolonic tissue (stolons) that extends from the basal area of the adult zooid (figure 21) (Gutierrez and Brown, 2017). Stolonic budding occurs at regulated intervals along the stolons, which helps to identify it from other sporadic types of asexual budding (figures 10. B and 11, bottom left) (Brown and Swalla, 2012).
3. Vascular budding
These buds form from the vessels that connect zooid individuals together (Gutierrez and Brown, 2017). This process involves the vascular epithelium enclosing the cell-rich blood at scattered sites along the interconnecting vascular network between zooids (Brown and Swalla, 2012). This in itself is particularly amazing as it requires multiple zooid individuals to orchestrate the unidirectional blood flow between themselves (Kalk, 1970). Vascular budding can be differentiated from stolonic budding by it's random and sporadic nature of growth (figures 10. A and 11, bottom right) (Brown and Swalla, 2012).
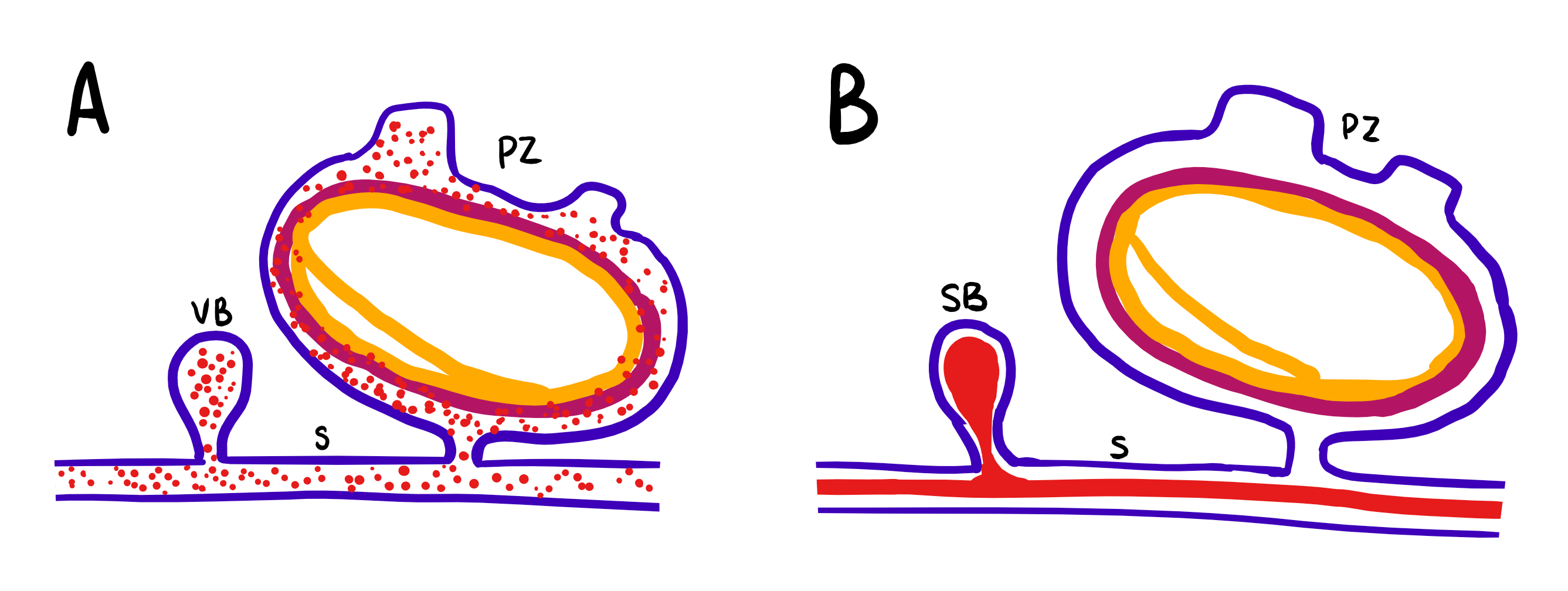
Figure 10: Schematic drawing of the differences in asexual budding of S. brakenhielmi; Blue = ectoderm, mauve = mesoderm, yellow = endoderm, red = blood, VB = vascular bud, SB = stolonic bud, S = stolon, PZ = parental zooid. (A) Vascular budding: buds are formed by sporadic enveloping of the epithelium, which enclose blood filled with hemocytes (stem cells). (B) Stolonic budding: buds are formed periodically at stolon intervals where blood is aggregated. Adapted from Brown and Swalla, 2012.
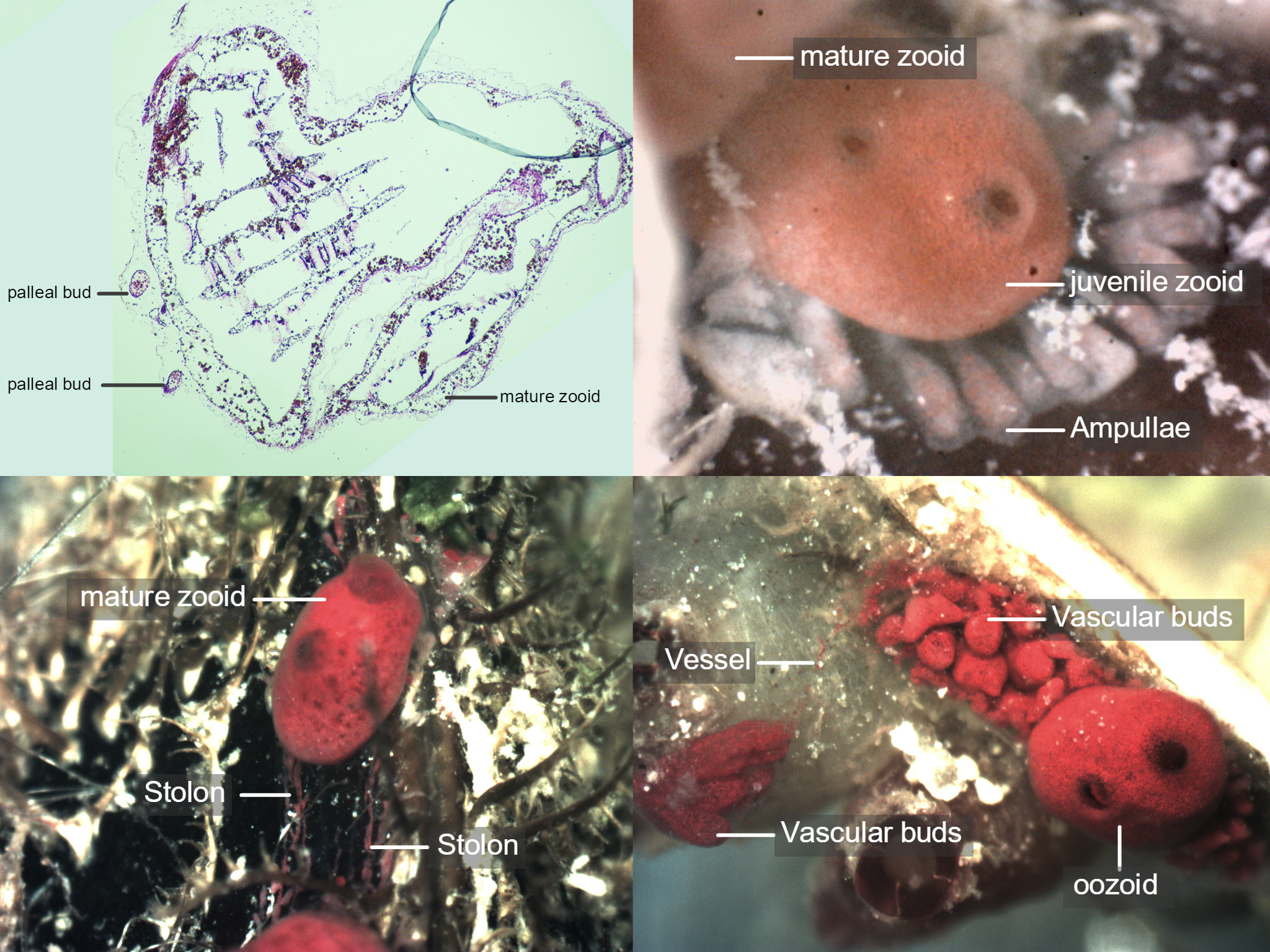
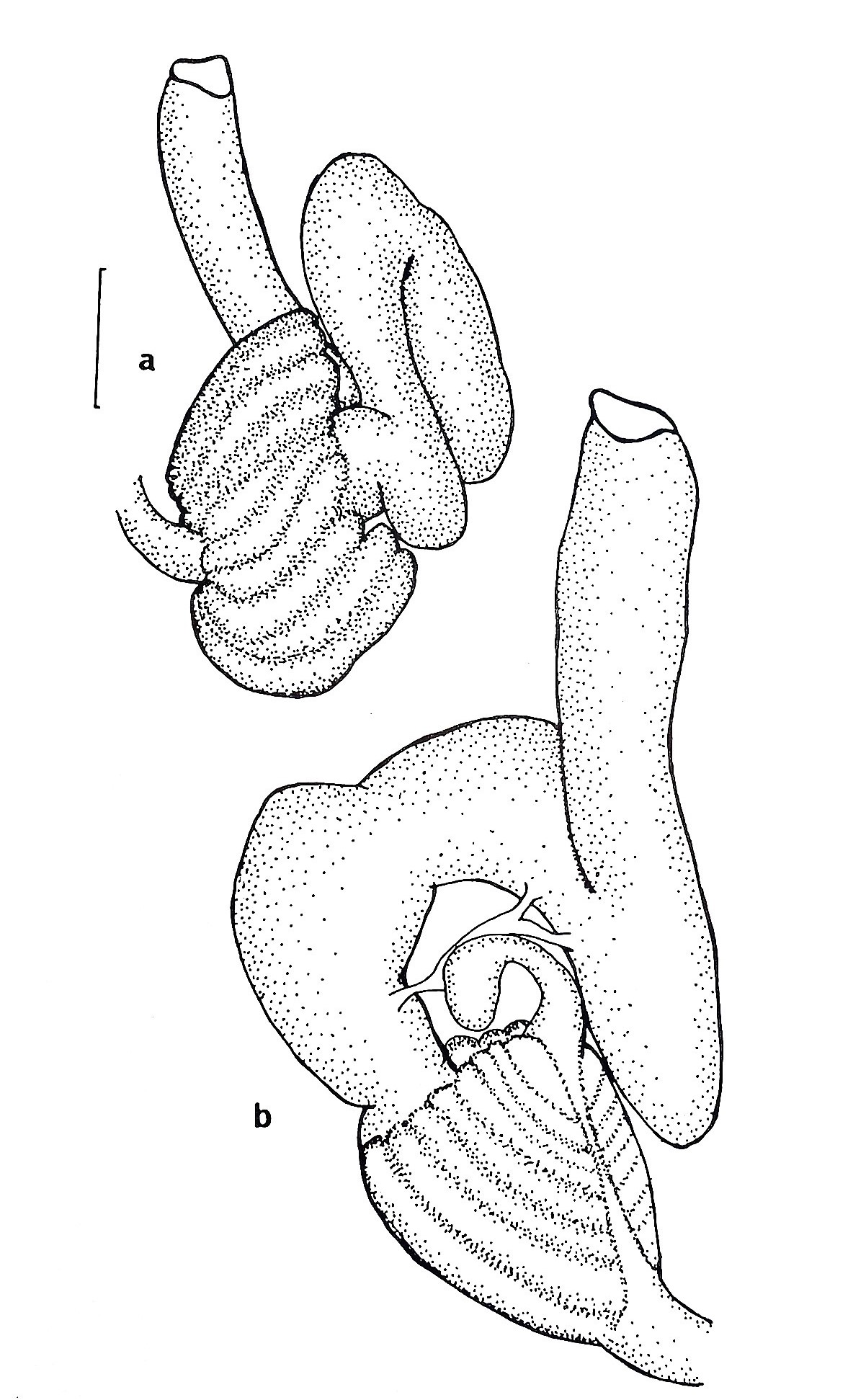
Figure 11: S. brakenhielmi can asexually reproduce via three different mechanisms; Pallial budding (top left) is when new buds form from the lateral walls of the parent zooid. Stolons (bottom left) are fibrous tubes that connect adult zooids together and allows the colony to share materials and fluids as well as undergo stolonic budding - where the intervals (nodes) of stolons swell and form new buds that then develop into zooids. Ampullae (top right) are not to be confused with stolonic buds, instead they function only at the periphery of the colony and assist with blow flow regulation. Vascular budding (bottom right), where the tunic of the parental zooid (oozooid) extends and fills with vessels which pump out cellular-rich blood into the tunic. The hemocyte cells differentiate and combine to make tissues and form new buds. Here we can see a vessel (not a stolon) that connects the two groups of developing zooids together. Also pictured are other species of ascidiacea.
Video 1: Example of vascular budding and unidirectional blood flow in Symplegma brakenhielmi. Buds are formed from enclosing epithelial tissue around cellular-rich blood. Undifferentiated cells form tissue and irregular buds within a common tunic. Vessels connecting buds and zooids together are distinguished from stolon by thin tubes, without intervals (Brown and Swalla, 2012).
Development
During the vascular budding process, the vascular epithelium envaginates to form a hollow sphere, and two layers are formed by the closing of the inner epithelium and the extension of the outer epithelium (figure 12; A to D). After the bud has formed, it then starts to go through the 7 different morphological stages to develop as an adult zooid (Figure 12; 1 to 7) (Gutierrez and Brown, 2017).
1. Internal vessicles and atrial tissue begin to fold, small endostyle forms, and the zooid begins to grow in size.
2. Pharynx is formed and oral siphon, blood vessels and ganglion develop.
3. Endosytle and atrial siphon are evident.
4. Heart beat begins, and the gut and pharyngeal slits are formed.
5. Stomach begins to fold, and stigmata are perforated.
6. Zooid reaches functional maturity; 8-9 rows of stigmata become active and apertures open.
7. Zooid reaches sexual maturity; Gonads form.
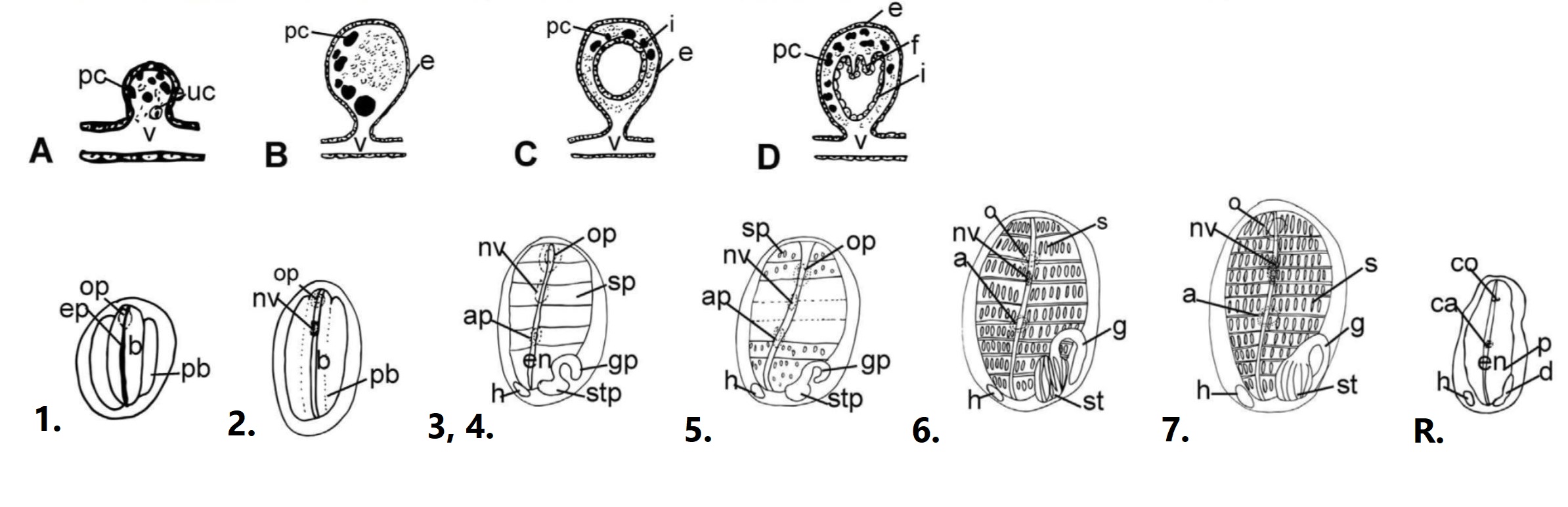
Figure 12: Development of a S. brakenhielmi zooid; Vascular bud forms - A to D show the envagination of the epithelial vessel forms a vesicle (bud). Development of the zooid - 1 to 7 show the seven stages of development from a juvenile (1) to a sexually mature zooid (7). Re-absorption (R) is the final stage of the zooid's life (12 days after initial bud forms). Important abbreviations: a = atrial siphon, ca = closed atrial siphon, o = oral siphon, co = closed oral siphon, p = pharynx, en = endostyle, f = folds, g = gut loop, st= stomach, h = heart, i = internal epithelium, nv = neural vesicle (ganglion), s = stigmata, v = vessel, uc = undifferentiated cells. Adapted from (Gutierrez and Brown, 2017).
Re-absorption and Recycling
The final step is unique to members of the Styelidae - zooids of a colony undergo re-absorption approximately 12 days after budding (figure 12; R.) (Gutierrez and Brown, 2017). The process starts by the closing of the siphons (no longer filtering), then a reduction in body size. The branchial basket degrades into single cells and the heart beat slows to a stop. The final step is re-aborption of cellular materials through the stolons into the adjacent zooid (Gutierrez and Brown, 2017). The majority of cell-death in a zooid is programmed into their genome and genetic switches regulate the re-absorption of zooid material (Kürn, et al, 2011). The degrading of zooid cells is also done by phagocytes/macrophage-like cells and generation of new buds is assisted by hemocytes (stem cells) that circulate in the blood, which then differentiate to produce tissues (Kürn, et al, 2011) (Gutierrez and Brown, 2017).
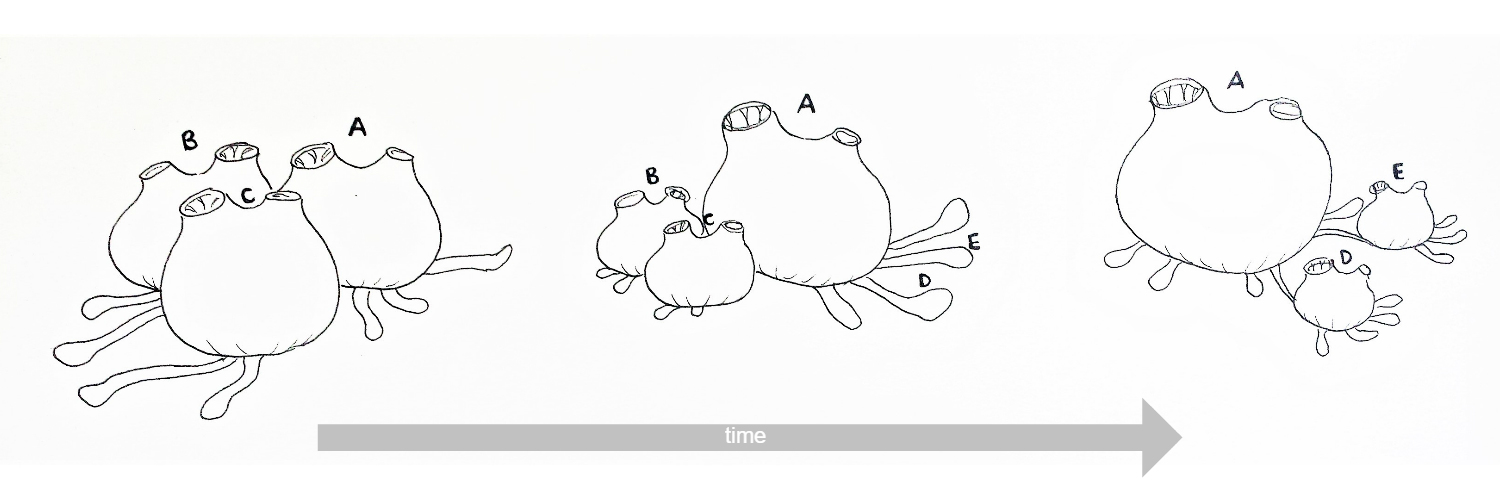
Figure 13: Schematic drawing of the re-absoption and recycling of zooids in S. brakenhielmi over time. Zooids B and C reduce in size and degrade, whilst zooid A grows larger (superzooid)and extends stolonic growth to the right, where new stolonic buds form zooids D and E (Gutierrez and Brown, 2017) (Kürn, et al, 2011). Mature zooids are 3mm wide.
It was observed from the samples used in this project, that the zooid that had re-absorbed an adjacent zooid's biological material, then grew in size (from 3mm to 4mm wide) (figure 13). Current research calls this phenomenon the 'superzooid' - a zooid that acts as a storage centre for a colony-wide recycling system (Kürn, et al, 2011). The superzooid then move materials of dying zooids into new buds in order to grow the colony (Kürn, et al, 2011). Unlike zooid regulation in Botryllus (sister taxon), the development and turnover of Symplegma zooids is asynchronous and the zooids work independently; several stages of zooid development and re-absorption can be observed within one colony at any given time (figure 14) (Gutierrez and Brown, 2017).
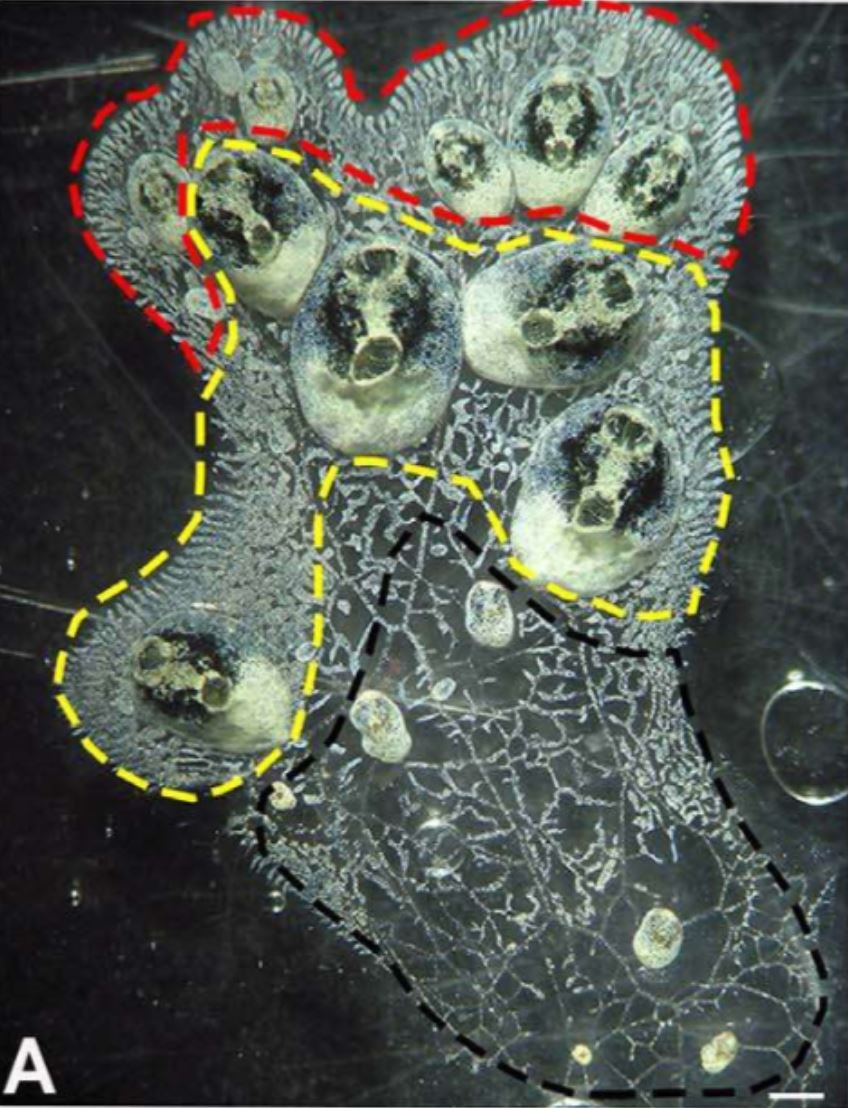 Figure 14 (left): White-green morph of S. brakenhielmi colony
that shows three different zonations of the colony;
red zone = budding zone where new buds are formed,
yellow zone = mature zooid zone (parents of buds), black
zone = re-absorption of zooids. Scale bar: 1mm.
Image source: Gutierrez and Brown, 2017.
Back to top of page
Figure 14 (left): White-green morph of S. brakenhielmi colony
that shows three different zonations of the colony;
red zone = budding zone where new buds are formed,
yellow zone = mature zooid zone (parents of buds), black
zone = re-absorption of zooids. Scale bar: 1mm.
Image source: Gutierrez and Brown, 2017.
Back to top of page
|
 |
| Figure 1 |
|
 |
| Figure 2 |
|
|
|
Sexual Reproduction | |
All ascidian species are hermaphroditic, meaning they have gonads that produce eggs and sperm within the one individual (Ruppert, et al, 2004). This trait has evolved primarily due to their sessile lifestyle, and inability to move around to find a mate to fertilise eggs with. Because S. brakenhielmi colonies are primarily asexually reproducing (Gutierrez and Brown, 2017), this species has evolved a trade-off in its sexual reproduction; they are ovoviviparous (brooding) and the ovary produces only 2-3 eggs at a time (Kott, 1985). This way, a colony can invest more into producing asexual clones rather than new individuals. If the conditions are right, right on dusk, zooids will cast sperm into the water coloumn. Simultaneously, a genetically different zooid can obtain sperm through filtration; the sperm travels to the atrium where unfertilised eggs are pushed out from the genital duct to be fertilised (Monniot et al. 1991). The zooid then broods the embyros in it's atrium, after which a lecithotrophic tadpole larva will emerge and leave the parent to find a place to settle and start a new colony (see Life Cycle) (Kott, 2004). Because the larva do not feed, their life in the plankton is very short, resulting in a shorter dispersal distance and low radiation of the colonies genes (Kott, 2004). To avoid self-fertilisation, ascidians have evolved species-specific gamete recognition mechanisms in their eggs and sperm (Murabe and Hoshi, 2001).
Although there has not been an in-depth study into the sexual life history of S. brakenhielmi, information gained from studies of closely related species, such as Symplegma reptans (Japanese colonial ascidian) can assist with understanding how S. brakenhielmi reproduces. Like most ascidians, Symplegma species can sexually reproduce year-round (Sugimoto, and Nakauchi, 1974).
The fertilised egg then undergoes the deuterostomic development into a larva; radial cleavage, blastrulation (formation of blastocoel), gastrulation (formation of through gut) and neuralation (Ruppert, et al, 2004). Ascidiacea do not have a true coelom (Ruppert, et al, 2004).
Tadpole Larva
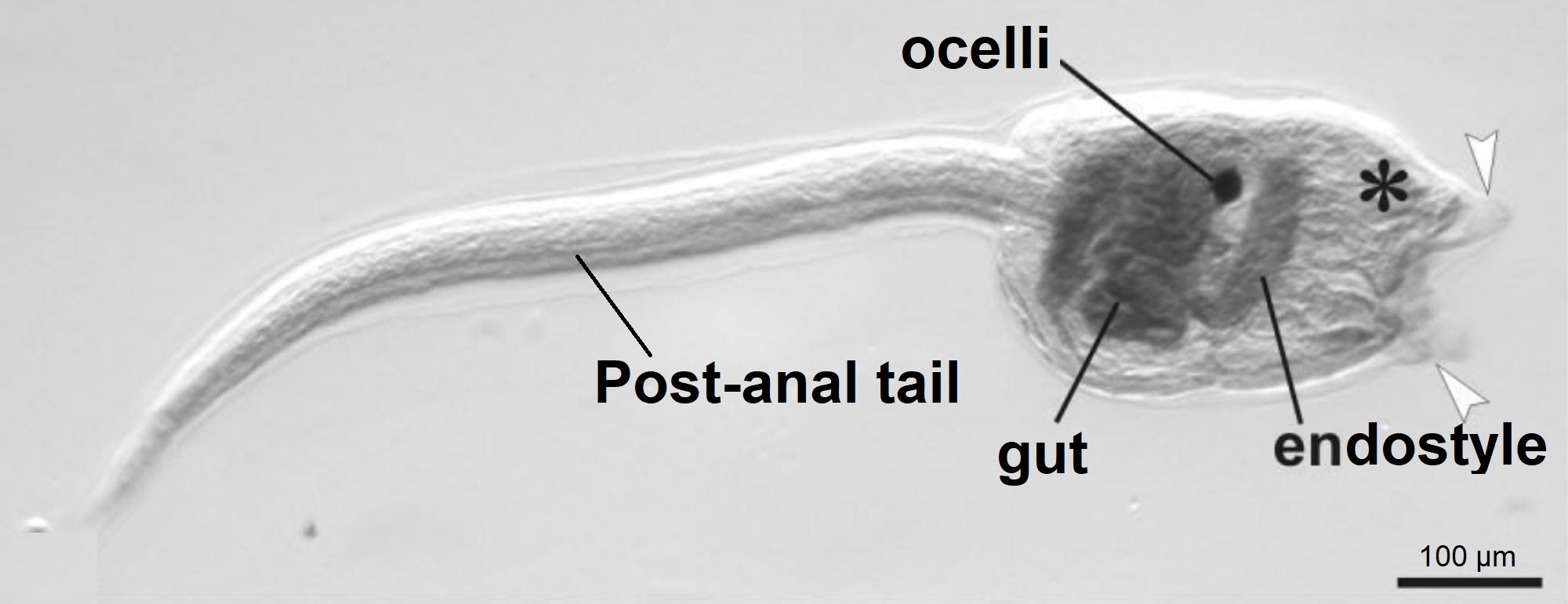
Figure 15: Botryllus schollerossi (compound Styelidae ascidian sp.) tadpole larva. Image shows the post-anal tail, gut, endostyle and ocelli (pigment cup). White arrows indicate where the three adhesive papillae (anterior appendages) are located, and the asterisks shows the location of emerging ampullae (Caicci, et al, 2010). Scale: 100 µm. Adapted from Caicci, et al, 2010.
Figure 15 shows the lecithotrophic tadpole larva of a compound ascidian, Botryllus, within the same family (Styelidae) as Symplegma, as no photographs of Symplegma larva have been published. Styelidae larva have a post-anal tail, which supports a hollow dorsal nerve cord and the notochord (chordate feature), and is used to help the larva swim through the water column (Caicci, et al, 2010) (Maliska, et al, 2013). In the trunk (head) there is a ganglion (brain) and a sensory vesicle which contains a statolith for gravity detection and ocelli for light detection (Monniot, et al, 1991). Lecithotrophic larvae do not feed, instead they have a large yolk sac (rudiment) which they use to gain their energy for swimming in dispersal (Ruppert, et al, 2004).
Metamorphosis
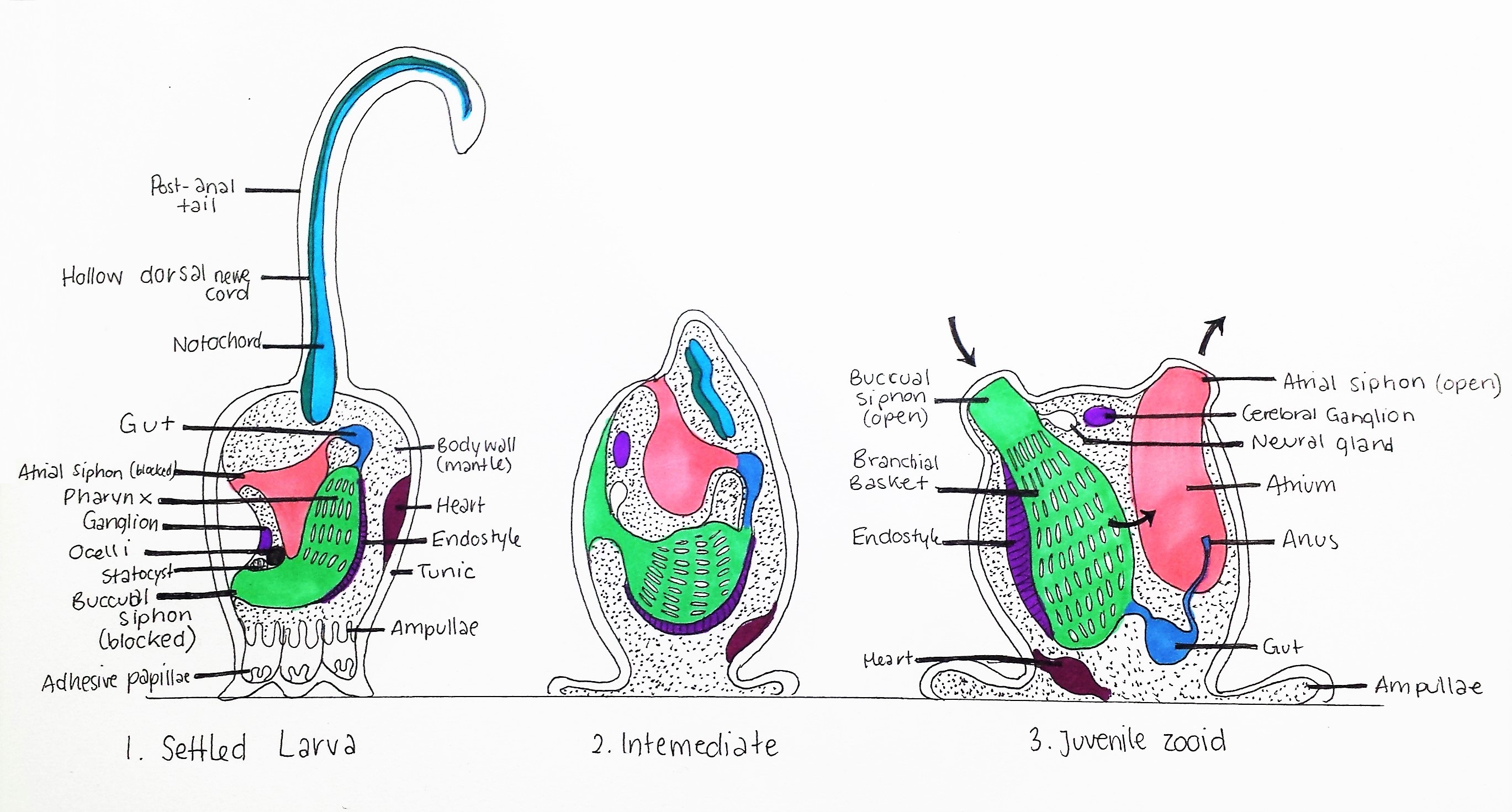
Figure 16: Metamorphosis of Styelidae larva; 1. Settled Larva: Tadpole larva attaches to substrate using three adhesive papillae, Notochord, hollow dorsal nerve cord and post-anal tail are attached to posterior, buccual and atrial siphons are blocked and statocyst and ocelli are intact. Pharynx (pharyngeal slits) is small.
2. Intermediate: Notochord, hollow dorsal nerve cord and post-anal tail are degraded, siphons migrate to the now dorsal side of the animal. Pharynx enlarges and amplullae spread. Ocelli and statocyst are degraded. 3. Juvenile zooid: Siphons open, pharynx enlarges further to form a branchial basket (see Anatomy and Physiology), heart migrates to the basal area, and adult neural gland and cerebral ganglion are present. Hollow dorsal nerve cord and Notochord are absent. Black arrows indicate the direction of water current generated by cillia in the pharynx. Adapted from Ruppert, et al, 2004.
The hatched larva of colonial ascidians like Symplegma are large and spend a very short time in the plankton, meaning they don't travel very far from the original colony (Dias, et al, 2006). After hatching, the larva use their ocelli to detect light at the surface of the water, and then swim down towards the substrate (Monniot, et al, 1991). Using their chemosensors in their trunk, the larva determines the safest and most optimal place to settle (free from toxic secretions), and the statocyst is used to orientate their body downwards (Monniot, et al, 1991). The three adhesive papillae attach to the substrate and the post-anal tail begins to degrade (figure 16) (Monniot, et al, 1991). The larval metamorphosis (figure 16) then follows with a 90º rotation of the animal within the tunic and enlargement of the pharynx into a branchial basket for filter-feeding (Ruppert, et al, 2004). The chordate larval features (notochord, hollow dorsal nerve cord, ocelli and statocyst) are degraded and the adult nervous system develops (Monniot, et al, 1991). These morphological larva features are the most significant evidence of the ancestral link between Urochodata and Vertebrates (see Evolution and Systematics)(Monniot, et al, 1991).
Back to top of page
|
 |
| Figure 3 |
|
|
|
Reproductive Strategies | |
When comparing the invasive success of S. brakenhielmi and S. rubra, it appears that the former has a much greater global distribution than the later (Rocha and Costa, 2005). A study by Dias, et al (2006) showed that S. rubra (close relative of S. brakenhielmi) has a very low dispersal distance due it's high preference for colonial budding (asexual) and low preference for sexually reproducing, which produces low dispersal lecithotrphic larva (see Sexual Reproduction). The two species have very similar ecological traits and both originate from the oceans surrounding central America; Gulf of Mexico and the Caribbean sea (see Biogeographic Distribution) (Dias, et al, 2006). Although the species is widely distributed around the coast of Brazil, due to their high preference for asexual budding over sexual reproduction, local populations of S. rubra have very low genetic variability between them (Dias, et al, 2006), making them very susceptible to large environmental fluctuations in temperature and salinity (Dijkstra, et al, 2008).
It has been proposed that both of these species are able to attach to floating man-made objects such as plastics, which then drift to new locations where a new colony can form (Dias, et al, 2006) (Kott, 2004). It could be inferred that this would not be a favoured method of dispersal for a soft-bodied ascidian species such as these two, drifting plastics often float into poor quality water filled with pollution and debris making it difficult to filter-feed efficiently. Thus, because S. brakenhielmi has a larger global distribution, it must have evolved a reproductive strategy that allows it to survive through stressful conditions and grow a new colony.
Asexual Reproductive strategies of S. brakenhielmi
To investigate which asexual reproductive strategy S. brakenhielmi prefers in a stressful environment, specimens that were found settled on a mussel clump from North Stradbroke Island, Queensland, were observed over seven weeks. Their asexual reproductive strategy preference was tracked after experiencing a stressful situation (being removed from a natural environment into an aquarium). The colony was first observed on the 14th of April, 2017, after being held in an enclosed tub with other invertebrates from the area for 1-2 weeks. Figure 4 shows a image of the colony when they were first observed (n=23 adult zooids), their colouration was a bright pomegranate-red and there appeared to be no extensive budding of any kind. The colony was then extracted from the large mussel clump until there were just three mussel shells with zooids attached (figure 7). The colony was then placed into an aquarium with other organisms and left over a 2 week period.
On return (week 4), the colony was observed to see what proportion of the colony (individual zooids) had adopted particular asexual reproductive strategy; stolonic budding or vascular budding. Palleal budding was not investigated in this study due to the ambiguity of it's identification. If the zooids were not asexually reproducing, this was also recorded. These observations continued for another three weeks and were recorded as proportions.
Results
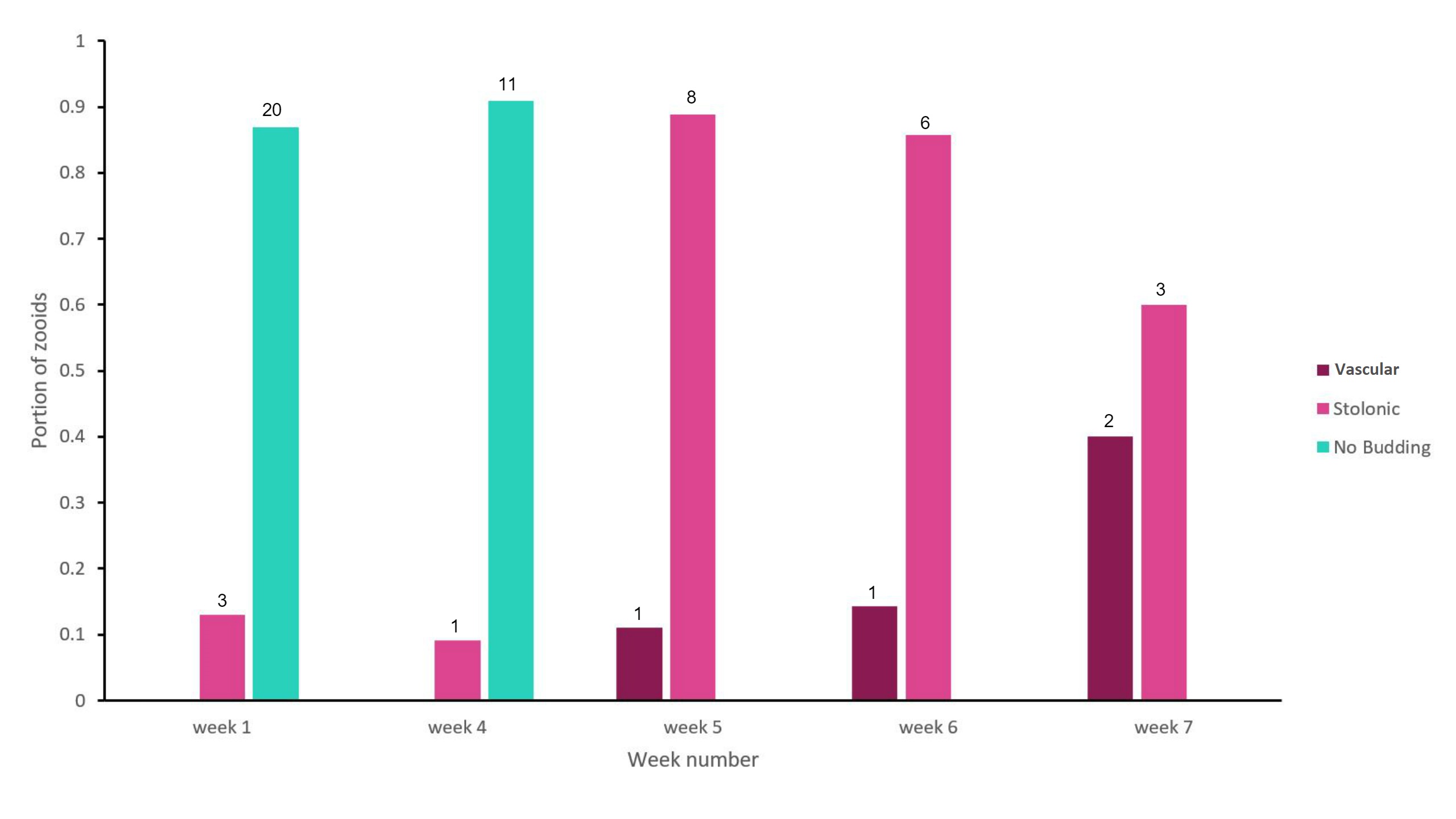
Figure 17: Bar graph showing the proportion of adult zooid S. brakenhielmi that showed a preference towards a particular asexual strategy; Vascular Budding or Stolonic Budding. No budding was also a preferred strategy of the zooids and is also represented in the results. The data was collected over 7 weeks, with weeks 2 - 3 left unrecorded. Mauve = vascular budding, pink = stolonic budding and green = no asexual budding occurring. The sample size for each preference for each week is recorded above the bar, and the sample size for each week (nw) are as follows; n1 = 23, n4 = 12, n5 = 9, n6 = 7, n7 = 5.
The results (figure 17) show that there was an alternating preference towards asexual reproductive strategy. For the first four weeks, the colony was not undergoing asexual budding. In the first week, the colony had not been introduced into the aquarium, so it seems that they were functioning as a stable colony and not needing to grow further (low space available on the shell surface). But the following three weeks are less obvious, as the amount of adult zooids reduced from 23 down to just 12. The colony has possibly triggered a stress response to the artificial environment, and forces the zooids on the edge of the colony to undergo re-absorption, creating superzoids (see Asexual reproduction). After four weeks of observations, the colony reduces further in size to 9 adults zooids and its preference for asexual reproduction increased. In week 5, the colony was dominated by zooids that produced stolonic buds and a small portion producing vascular buds. Over the last two weeks of the observational study, the ratio of stolonic budding and vascular budding evened out, but the number of adult zooids still decreased. In Dias, et al (2006) study, they found that S. rubra had cloned genotypes within a population, which genetically reinforced asexual reproduction. For the S. brakenhielmi specimens, asexually reproduction seemed more advantageous to their survival because they were in a new environment and were isolated from genetically different colonies. This is important to consider, as ascidians are hermaphrodites and have strong biological controls that stop self-fertilisation (Murabe and Hoshi, 2001), if there are no other colonies around to reproduce with, there would be no point to sexually reproducing. Furthermore, environmental conditions are believed to have the most significant effect on the ratio of asexual budding to sexually reproducing within a colony (Dias, et al, 2006). So it is likely that these specimens are using information from their environment to conserve biological material (through re-absorption) and wait until they are in more favourable conditions to grow. It was observed that there were zooids using vascular budding to produce large quantities clones contained within a common tunic (figure 11; bottom right, 18, video 1, ). Although there is no research published to explain this variation of vascular budding, it could be that the colony is 'waiting' for more favourable, release large amounts of underdeveloped zooids and then grow the colony size very quickly.
S. brakenhielmi's invasive success over S. rubra is likely to be linked their ability to re-absorb and recycle zooids, choose the best reproductive strategy (or alternate between) and survive long periods of time whilst attached to drifting debris and marine vessels (Dias, et al, 2006). Future research should be investigate the variations in vascular budding and how an invasive colonial ascidian species such as S. brakenhielmi respond to more realistic changes in their environment.
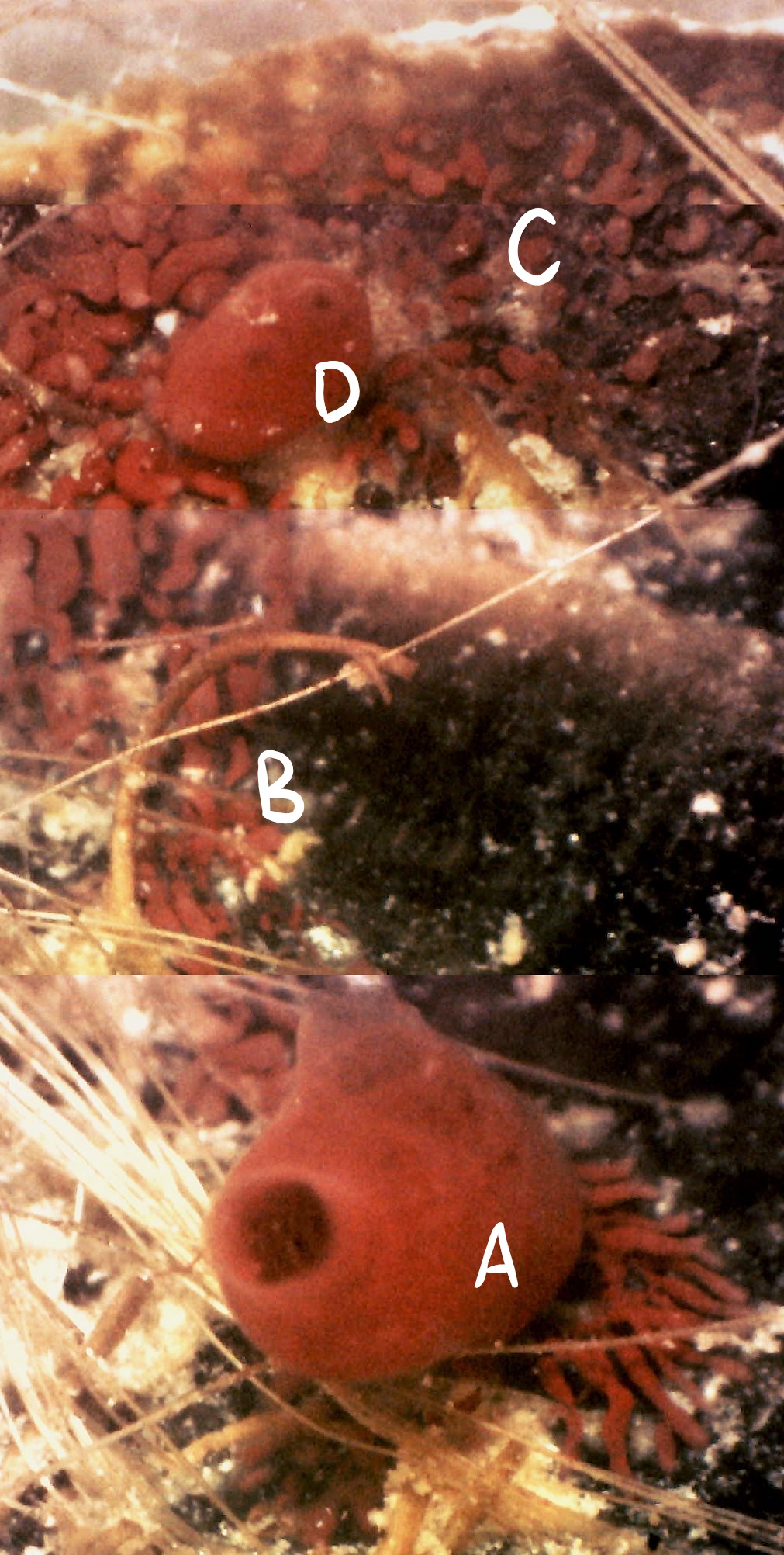 Figure 18 (left): Photograph taken during week 7 of the observational study; a parental zooid (A) that has created connective vascular buds (B) to a large vascular budding mat
Figure 18 (left): Photograph taken during week 7 of the observational study; a parental zooid (A) that has created connective vascular buds (B) to a large vascular budding mat
(C). A juvenile zooid (D) may also be assisting with budding. Vascular budding has a sporadic nature and is contained within
a common colonial tunic (see Asexual reproduction).
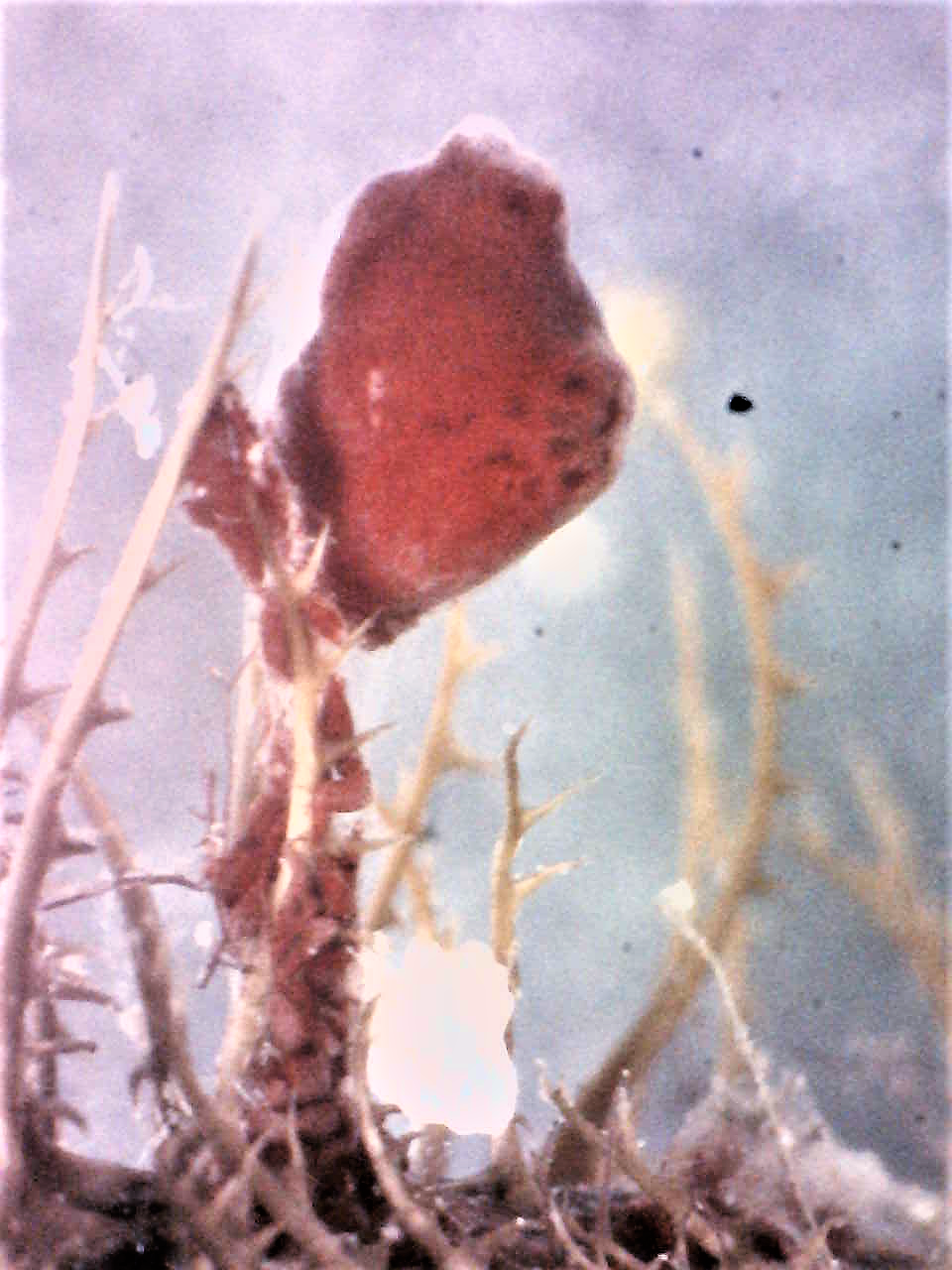 Figure 19 (above): Photograph taken during week 7 of the study, a
S. brakenhielmi zooid attached to the top of a piece of algae
that is growing on the shell of the mussel. The previous week, a larger zooid was positioned on the surface of the
Figure 19 (above): Photograph taken during week 7 of the study, a
S. brakenhielmi zooid attached to the top of a piece of algae
that is growing on the shell of the mussel. The previous week, a larger zooid was positioned on the surface of the
mussel, it is possible that by using vascular budding, the zooid moved materials to the top of the stem, so that a new bud
could form and gain the best space for efficient filter-feeding
and maximum food particle obtainment.
Back to top of page
|
|
|
Behaviour | |
 Filter-feeding Filter-feeding
The ascidians' secondarily derived sessile habit comes from their filter-feeding ancestry (see Evolution and Systematics) (Ruppert, et al, 2004). By generating a current using the cillia of their branchial basket (see Anatomy and Physiology), the tiny zooids collect food particles from the water column to eat (Ruppert, et al, 2004). They're food includes anything organic that is less that 100 µm - this includes plankton and algae (Rosa et al, 2013) (Ruppert, et al, 2004).
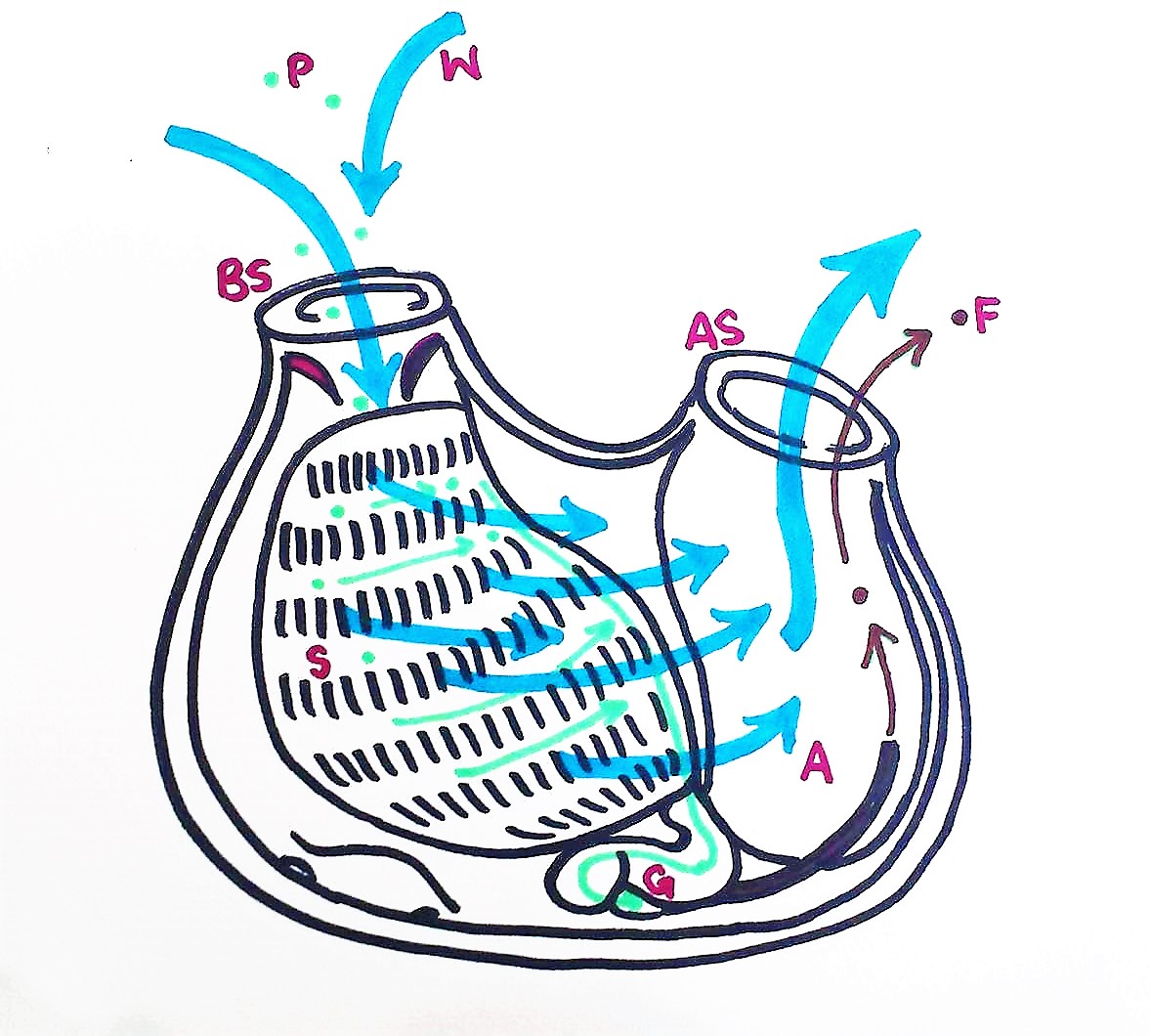
Figure 20: Schematic drawing of a S. brakenhielmi zooid filter feeding; Water enters the buccual siphon, into the branchial basket, through the pharyngeal slits and out of the atrial siphon. Food particles get trapped in the mucous net, and cillia push the food across the stigmata to a pharyngeal groove and down into the esophagus. The food particles are digested in the gut, and feces exits through the anus and is pumped out of the body via the excurrent through the atrial siphon. P = particle, W = water, BS = Buccual siphon, S = stigmata, G = gut, A = anus, AS = atrial siphon, F = feces, (Ruppert, et al, 2004).
Video 2: A S. brakenhielmi zooid positioned close to the surface of the water; the water and particles surrounding the zooid are drawn towards the buccual siphon (visible) by cililary action. Water that is drawn in through siphons is filtered for food by the branchial basket, before exiting through the atrial siphon (not visible) (Ruppert, et al, 2004). Here you can see the power of filtration from just one zooid.
|
|
|
|
Anatomy and Physiology | |
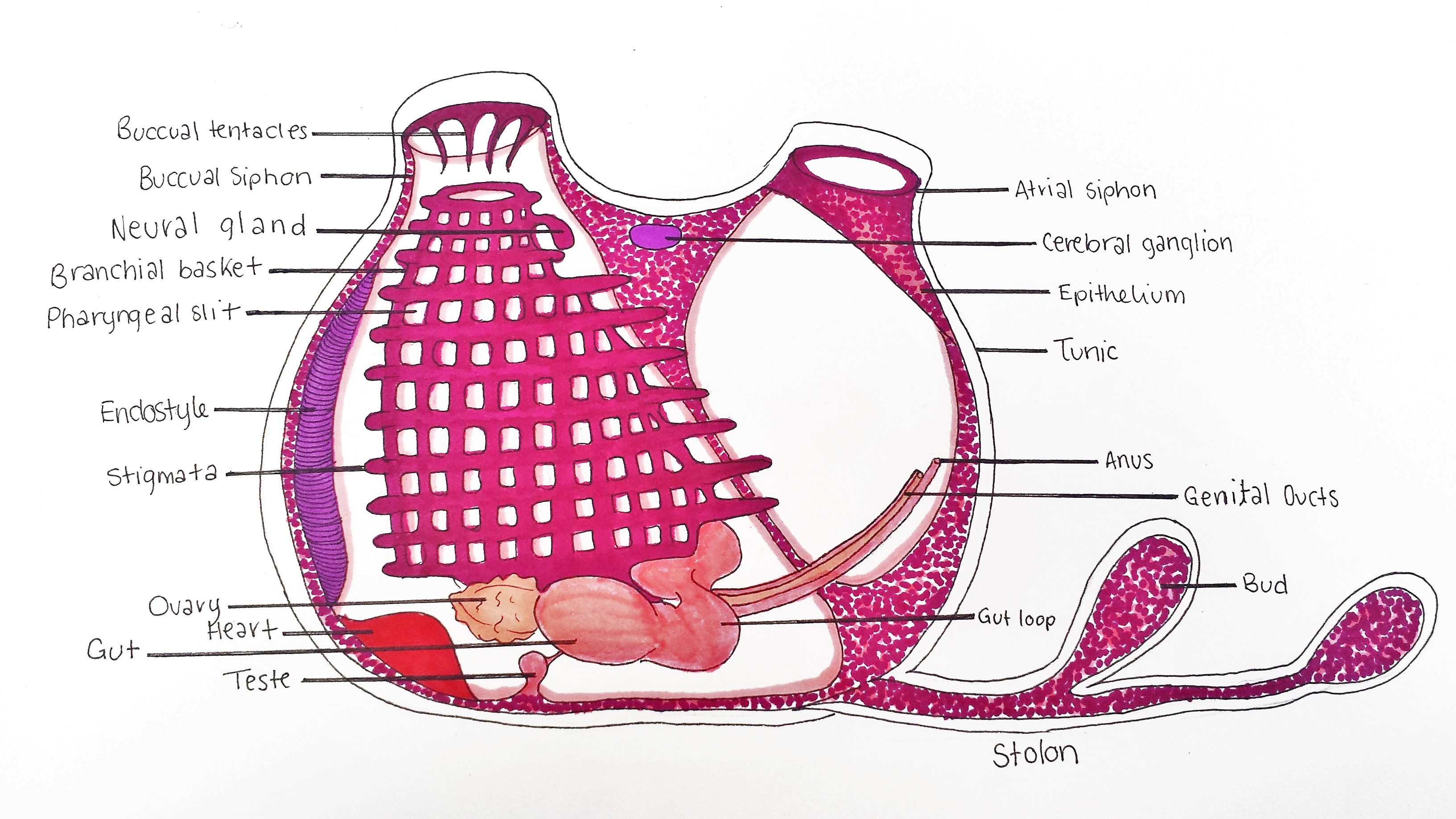
Figure 21: Schematic drawing of an adult S. brakenhielmi zooid. Buccual siphon supplies zooid with incurrent flow to the branchial basket (pharynx). Buccual tentacles prevent large particles from entering the basket, whilst the endostyle secretes a mucous web to capture small food particles. Tiny cillia on the stigmata move food toward the gut loop (esophagus), and then digest the food in the gut. Feces is excreted from the anus, which is expelled out of the artium by the excurrent of water through the atrial siphon. Small ovary has an anterior and posterior male follicle (testes) attached, and expel sperm and eggs from the genital ducts. A tubular heart pumps blood through the body wall (mantle), and into the stolons which are connected to the rest of the colony and growing buds. The Neural gland and cerebral ganglion form part of a reduced central nervous system. Zooid width ~ 2mm.
Branchial Basket
The pharynx also known as a branchial basket, of all ascidian species has been co-adapted for filter-feeding and respiration (Monniot et al. 1991). In figure 22, it is clearly represented by 10 to 11 rows of horizontal stigmata (species-specific), and multiple pharyngeal slits between each row (Kott, 2004). The top of the basket is attached to the mantle below the buccual tentacles (figures 21 and 26) and runs vertically down towards the base of the animal and exits into the esophagus (gut loop) (Monniot et al. 1991). The stigmata are covered in cilia that generate a strong current by beating, in which water is pulled through the buccual siphon (Monniot, et al, 1991). Larger particles are filtered from entering the basket by 12 buccual tentacles ( in S. brakenhielmi ), then the smaller food particles in the water current get caught in the mucus that covers the stigmata (Monniot et al. 1991). The mucus is secreted by the endostyle (figures 21 and 24) which is closely attached to the branchial basket, and runs along the left side of the body wall. The mucus is built into tiny webs, and once food particles are caught and pushed across into a dorsal mucus tract (pharyngeal groove) that then funnels down into the esophagus (figure 20) (Monniot et al. 1991).
The branchial basket is also the location where respiration occurs. Oxygen diffuses directly into the stigmata, which then migrates to cells around the body in the circulatory system for cellular respiration (Monniot et al. 1991).
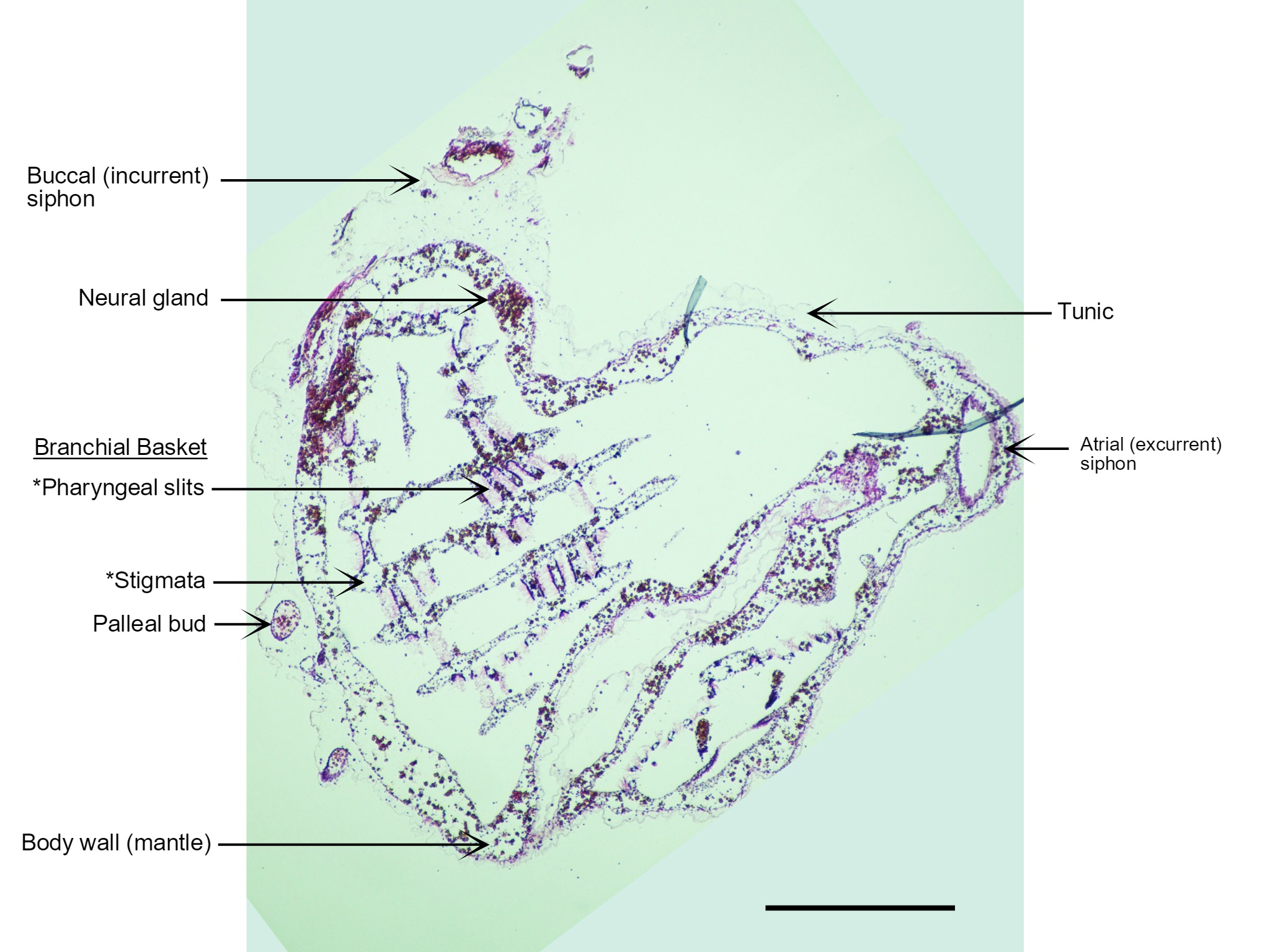
Figure 22: A section of a S. brakenhielmi zooid cut perpendicular to the base (posterior) of the animal and stained with H.E. Visible in this section are the two anterior siphons; incurrent (left) and excurrent (right), the cerebral ganglion, endostyle, branchial basket (and gill slits), a transparent tunic and a blood-filled body wall (mantle). Also captured are two small pallial buds that have detached from the large zooid and are insitu within the tunic. The large tube-like feature on the right side of the zooid that extends from the base to the excurrent siphon is actually just an infold in the body wall. Scale: 500 µm.
Tunic
The tunic of S. braknhielmi is a thin and soft outer tissue that has multiple layere (figures 21 and 22) (Ruppert, et al, 2004). To increase its rigidity, the tunic of the colony is composed of a living tissue made with cellulose-like fibres called tunicin (Ruppert, et al, 2004). These tunicin fibres are laid ontop of each other at different orientations to increase the strength further (Ruppert, et al, 2004). This species of ascidian also has tunic vessels, which form from outpocketings of the body wall, into the tunic. Hemocytes (blood cells) can be seen moving through the tunic and around the siphons using the vascualr tunic network (Ruppert, et al, 2004). Microsyimbionts also live in the tunic of some colonial species of ascidiacea, collecting light from the sun to produce food products used by the ascidian (Ruppert, et al, 2004). Although no current research has investigated if S. brakenhielmi has symbionts associated with its tunic.
Digestive System
S. brakenhielmi is apart Stylidae which all have their gut loop (figure 25) to the left side of their pharynx (branchial basket) (Kott, 1985), which helps to distinguish between families of ascidiacea. The gut loop is a circular esophagus which connects to a short and wide stomach (figure 23 and 24) (Kott, 1985), and is filled with more mucus-secreting/food absorbing cells (Monniot et al. 1991). The stomach as 10 folds and an a attached cecum which is curved around the gut loop (Kott, 2004). The intestine then extends to a rectum which is situated on the side wall and the anus opens up into the atrium (Kott, 1985). Feces moves out of the anus and is pushed out of the atrium by the excurrent of water flow (Monniot et al. 1991).
Figure 23 (right): (a) ventral view of the gut loop of Symplegma oceana (synonymous to S. brakenhielimi),
(b) dorsal view of the gut loop. Scale: 0.25mm (Kott, 1985). The shape position of the gut loop is used to differentiate closely related species from one another.
Excretory System
Unlike all other deuterstomes, ascidians have secondarily lost their nephridia (excretory organs), thus most species (including S. brakenhielmi) rely on the diffusion of ammonia and waste across the pharynx and other tissues (Ruppert, et al, 2004). The waste is then taken away from the body via the excurrent of water flow (Ruppert, et al, 2004).
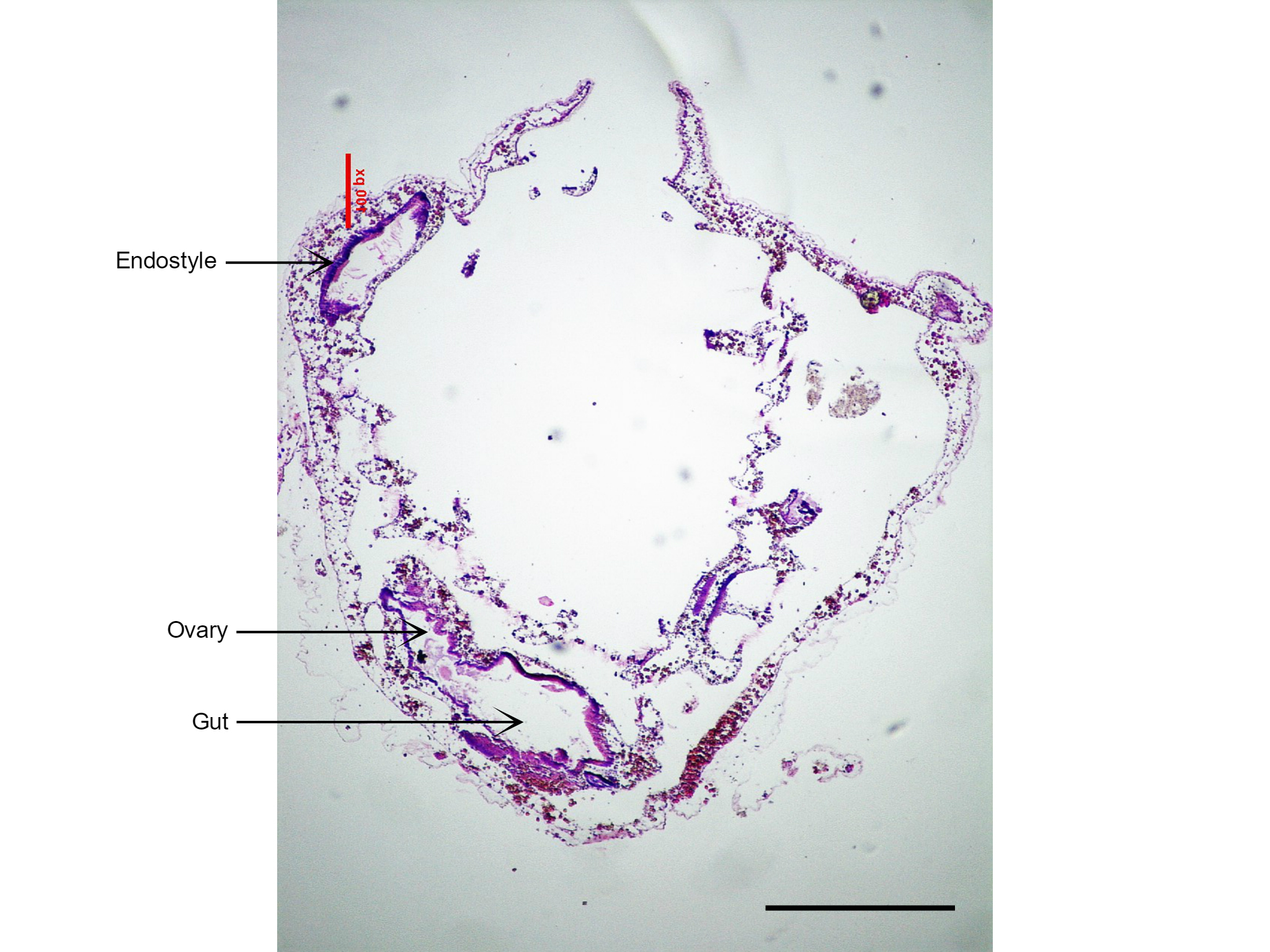
Figure 24: Section of S. brakenhielmi showing the short and large gut tube, and attached ovary (gonad). Also visible is the large endostyle to the side of the branchial basket, which secretes the mucus webs for filter feeding. Scale: 500 µm.
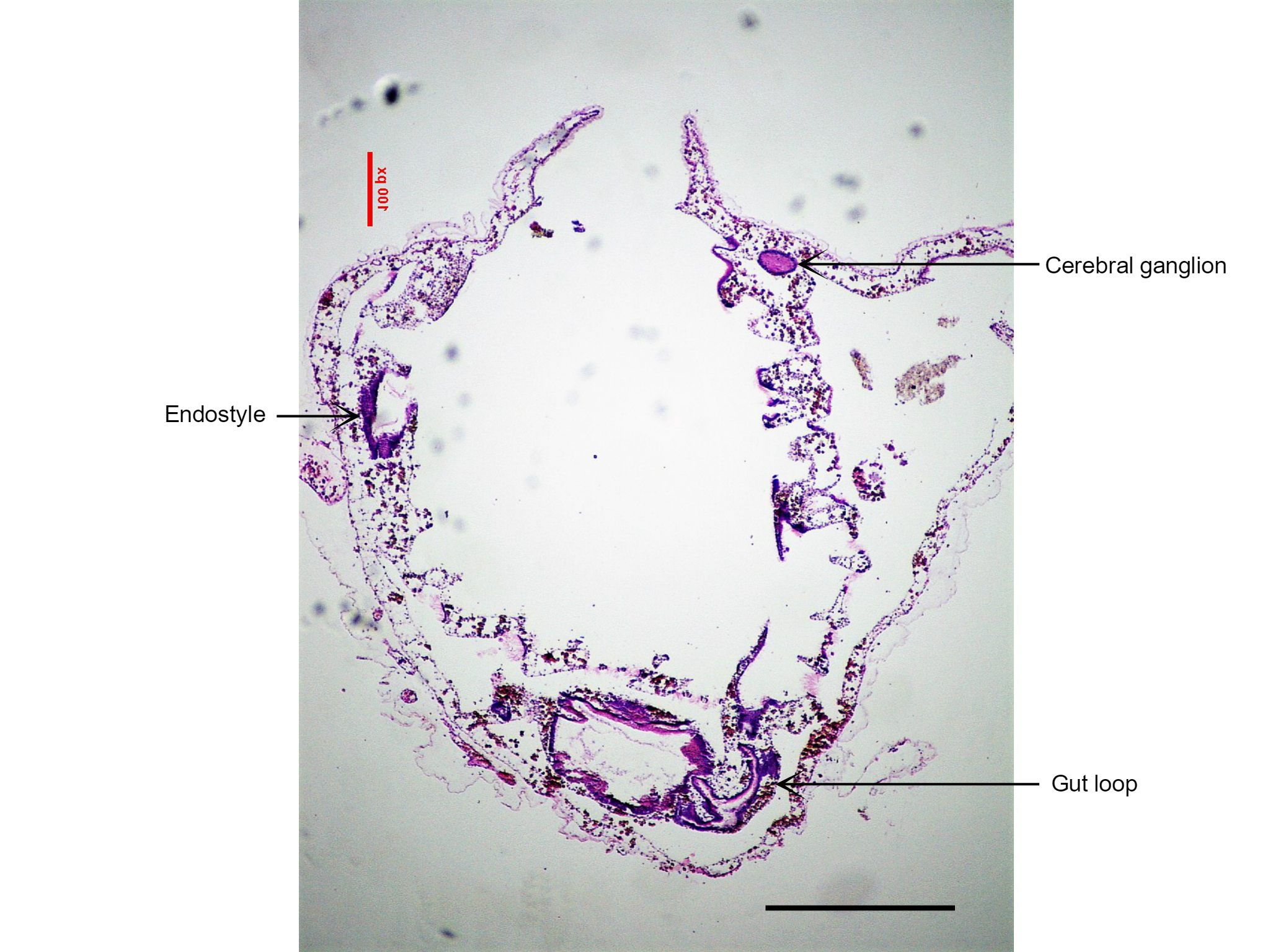
Figure 25: This section of S. brakenhielmi shows the cerebral ganglion (brain) of the zooid, as well as the gut loop used to distinguish clades of ascidians. Scale: 500 µm.
Nervous System
The cerebral ganglion and neural gland form part of the ascidian's reduced central nervous system (figure 21)(Monniot et al. 1991). The cerebral ganglion (figure 25) is situated on the dorsal side of the animal, in the body wall between the incurrent and excurrent siphons (Monniot et al. 1991). The ganglion is composed of a cortex and nerve cells that surround a fibrous central neuropile (Koyama and Kusunoki, 1993). On either end of the ganglion, there are nerves that extend toward each siphon, the anterior nerves controls the buccual siphon and associated musculature, whilst the posterior is associated with the rest of the body (Ruppert, et al, 2004). Interestingly, ganglion structure has been linked to the neural synapses of vertebrates (Koyama, and Kusunoki, 1993) (Lane, 1972). The neural gland is a hollow organ that is directly attached to the branchial basket via a ciliated dorsal tubercle (figure 21). It is responsible for regulating blood volume by injecting sea water into the circulatory system (Ruppert, et al, 2004). There is also some evidence to say that the neural gland may be involved in neural cell production by using environmental cues from the incurrent water to regulate production (Burighel, et al, 1998). Unlike other colonial invertebrates (bryozoans and cnidarians), the zooids of a S. brakenhielmi colony do not have interconnected neural network, thus each zooid can respond independently to stimuli (Mackie, 2005). Instead, electrical impulses are stimulated by the epithelial layer of the interconnecting blood vessels between zooids to assist with neural communication (Mackie, 2005). This epithelial tissue does not contain neurons, and instead works similarly to the impulses created by pacemaker cells in the heart (Ruppert, et al, 2004).
The central nervous system of the larval ascdian is vastly different from that of the adult, as their sensory systems are adapted to their lifestyle (pelagic and sessile respectively) (Mackie, 2005). Adult ascidians do not have ocelli, but still respond to differences in light by contracting their siphons when a shadow passes over (Mackie, 2005). Pigment cells around the siphons detect changes in light intensity, and cause a cross reflex (contraction) of the muscles (Mackie, 2005).
Circulatory System
All ascidian species have a specialised open circulatory system, in which a tubular heart (figure 26) at the base of the animal, pumps blood through the connective tissue (mesenchyme) of the body wall (mantle) (Monniot et al. 1991). Their blood is filled with metabolite products and undifferentiated hemocytes that produce multiple cellular products. These are essential for growth of the colony (budding): stem cells, lymphocytes, phagocytes, pigment cells and more (Monniot et al. 1991). Ascidians are also unique in that they have two pacemaker cells that alternate the direction of the blood flow, meaning it is unidirectional (video 1) (Kalk, 1970). The direction changes every couple of minutes, and is believed to be controlled by one dominant pacemaker cell that is sensitive to carbon dioxide concentration (Kalk, 1970). The S. brakenhielmi colony is actually a collection of zooids connected by an intimate vascular network (Mackie, 1983), hence, there must be a strong biological rhythm amongst zooids that allows them to regulate the direction of the blood flow. Swollen blind endings of the colonial vascular system are known as ampullae (figure 11, top right) - contractions of ampullae on the periphery of the colony assist with the changing direction of blood flow between zooids (Mackie, 1983).
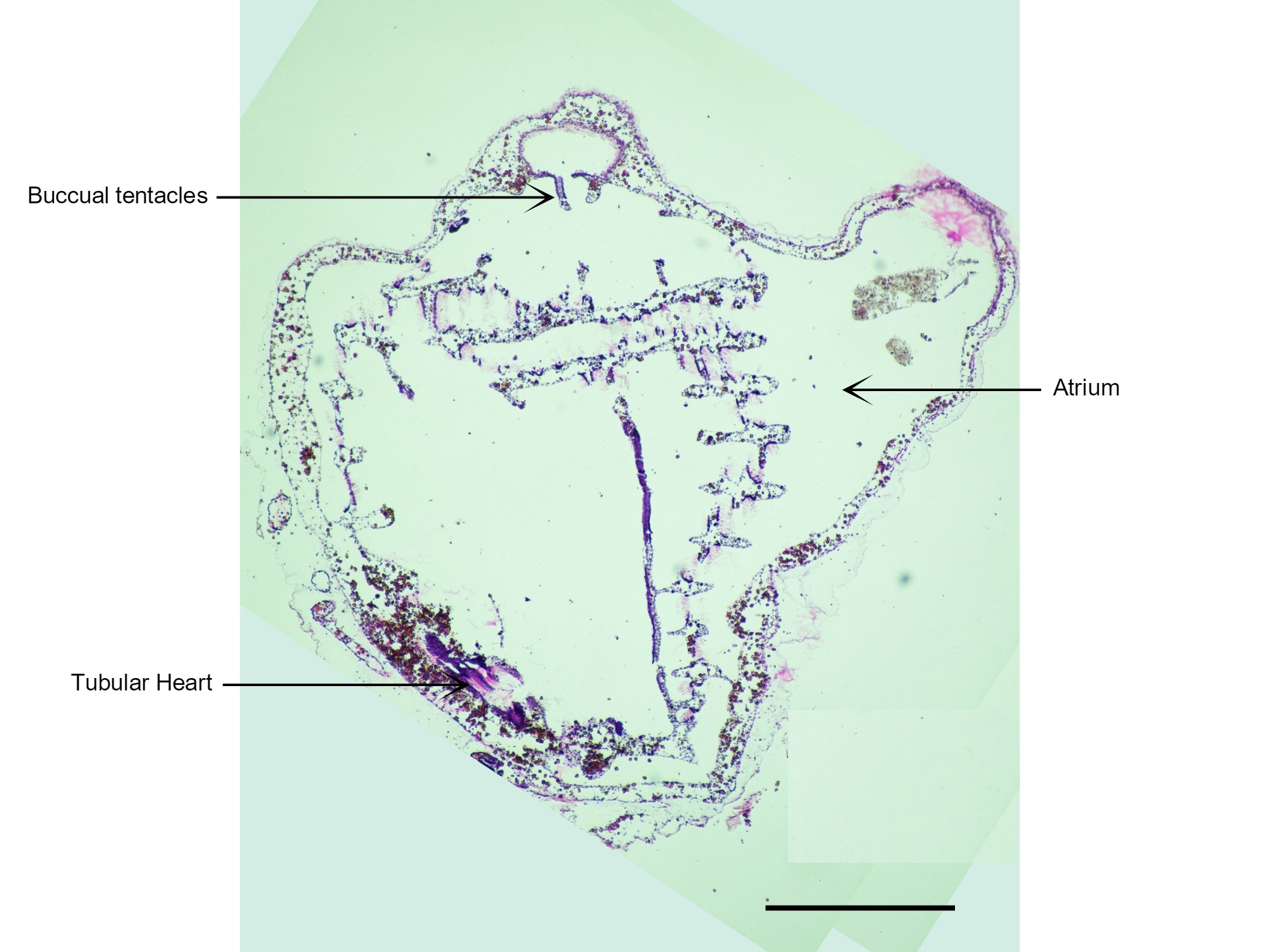
Figure 26: Visible in this section is the zooid's heart and buccal tentacles that are only found on the incurrent siphon. Scale: 500 µm.
Gonads
Because S. brakenhielmi is a predominantly asexual species, it has evolved specialised gonads for sexual reproduction. The ovary relatively small (figures 24 and 27) and only produces 2-3 eggs at a time and is adjacent to two small lobed male follicles (testes) (Kott, 1985). Fertilised eggs are brooded in the atrium were they develop and hatch as lecithotrophic tadpole larvae (see Sexual Reproduction) (Kott, 2004).
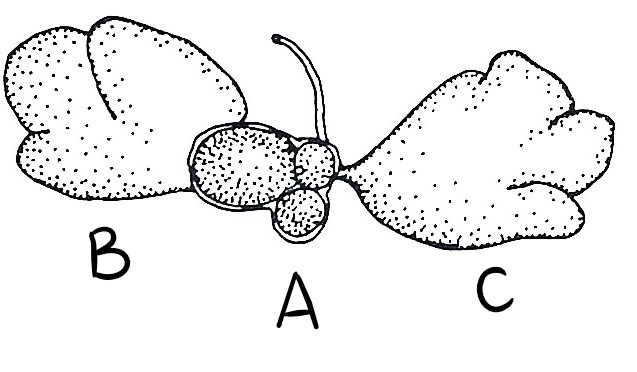
Figure 27: (A) Small ovary with 3 eggs, (B) Anterior male follicle and (C) Posterior male follicle (testes) (Kott, 1885).
Chemical Defences
A S. brakenhielmi colony is completely sessile and the zooids soft bodies are highly vulnerable to predation being settled on by other marine invertebrates (bryozoans, coral etc.). Adult zooids produce secondary metabolites and inorganic acids from their epithelium and are stored in the zooid's tissues (Pisut and Pawlik, 2002). Chemicals in the tunic protect the zooid from predation from grazers like crabs and fish, whereas chemicals stored in the viscera and gonads protect the eggs from specialist free-swimming flatworms (Pisut and Pawlik, 2002).
Recently, scientists have found that the secondary metabolites produced by species of Symplegma are economically valuable for pharmaceutical use (Youssef, et al, 2015). Purine and carboline alkaloids are a class of nitrogenous secondary metabolite specialised to Symplegma and have been show to have antimicrobial, enzyme inhibiting and cytotoxic properties and can even alter neural signals to the brain (Youssef, et al, 2015).
Back to top of page
|
 |
| Figure 4 |
|
 |
| Figure 5 |
|
 |
| Figure 6 |
|
 |
| Figure 7 |
|
 |
| Figure 8 |
|
|
|
Biogeographic Distribution | |
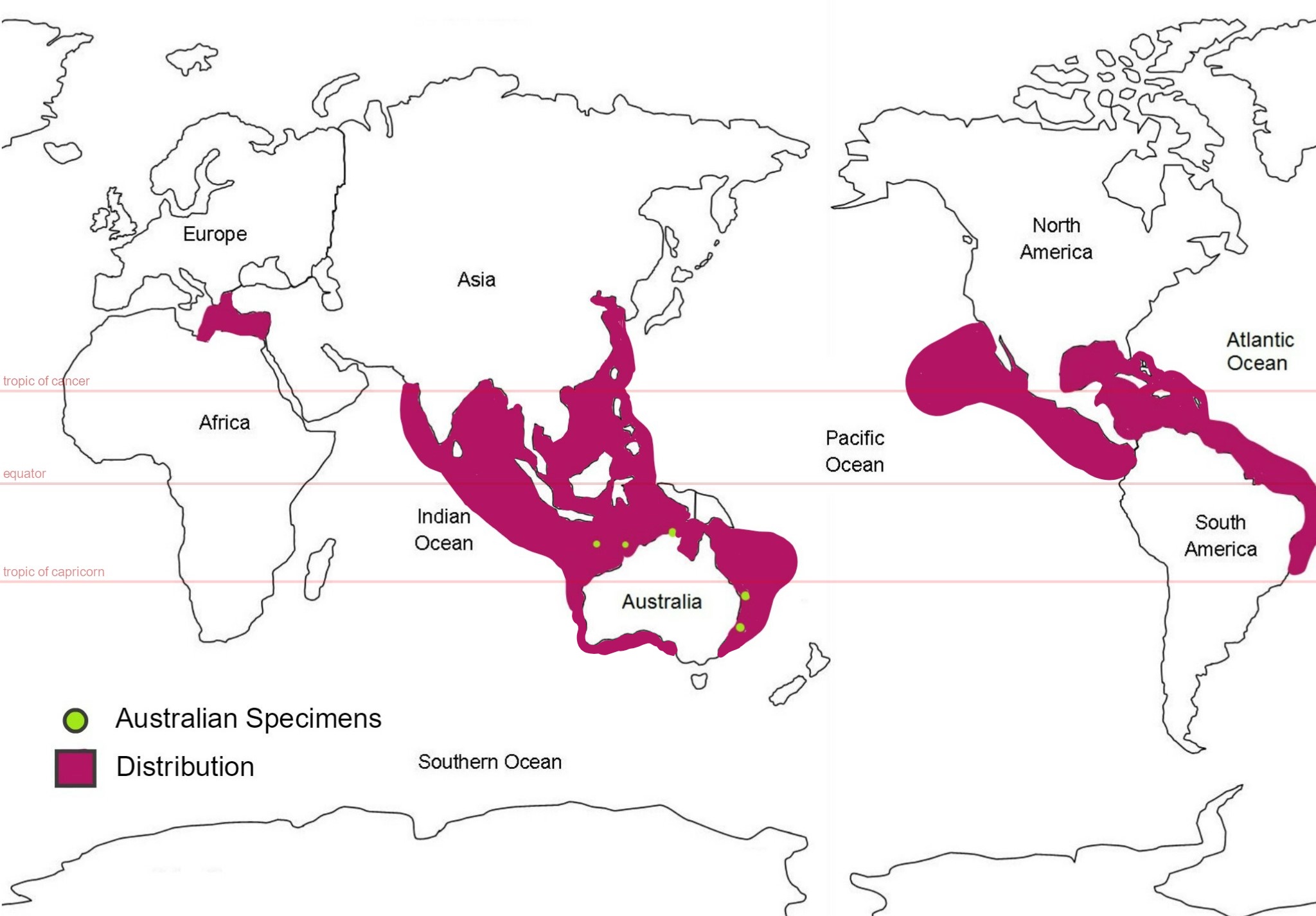
Figure 28: Global distribution map of S. brakenhielmi; mauve colour represents the estimated distribution and green circles represent areas where samples of the species were collected from in Australia. (Abdul and Sivakumar, 2007) (Kott, 1985) (Kott, 2010) (Kott, 2004) (Smithsonian National Museum of Natural History, 2017) (Lambert and Lambert, 1998) (Coles, et al, 1999) (Çinar, et al, 2006).
Global Distribution
This species is thought to have originated off the coast of Brazil and Panama has a similar distribution to its relative species Symplegma rubra, making it very difficult to distinguish between the two (Rocha & Costa, 2013). But it was first described by Michealson from a specimen in the Gulf of Mexico, in 1904. Records of its global distribution show that it lives only in warm sub-tropical/tropical water (pan tropical range) (Kott, 2004) and has invaded the southern coasts of India, the West Indo-pacific, including eastern Australian waters, as well as the Timor sea, West Indian Ocean, coasts of Asia, the Caribbean sea, the Pacific coast of North America, Hawaii, and the Mediterranean sea (figure 28) (Abdul and Sivakumar, 2007) (Kott, 2010) (Kott, 2004) (Smithsonian National Museum of Natural History, 2017) (Lambert and Lambert, 1998) (Coles, et al, 1999) (Çinar, et al, 2006). Their ability to survive through poor quality environments like ballast water means that they are able to invade foreign coastlines (Çinar, et al, 2006). They also require very minimal amount of surface area to stay attached to; a specimen was found attached to tiny piece of algae floating in the harbour of San Pedro Bay, California - researchers believe it was the first record of S. brakenhielmi on the Pacific coast of North America (Lambert and Lambert, 1998). It had likely arrived through either ballast water from South American ship or drifted over long distance via currents (Lambert and Lambert, 1998). Temperature and salinity are two of the main variables that restrict the distribution of introduced ascidians, as their physiology needs to be adapted to the conditions (Dijkstra, et al, 2008).
Australian Distribution
S. brakenhielmi was first discovered in Austalian waters by Patricia Scott, sometime between 1985 and 1992 , offshore from South East Queensland in Moreton Bay (Kott, 2010). Colonies were found on rocks at Dunwhich and Myora Banks on North Stradbroke Island, on wrecks at Cowan Cowan Moreton Island, on the seagrass mud flats Cleaveland Point and on the gritty mud flast Hutchinson shoals (Kott, 2010). The specimens that were worked on for this project were collected from North Stradbroke Island, to which Kott's observations were more than helpful in determining which species of Styelidae we had found.
The species was then discovered near Sydney New South Wales, in the Timor sea off the coast of Western Australia, in Darwin in the Northern territory, at Kangaroo island in the Great Australian Bite in South Australia, Heron Island, Townsville, Moreton Bay and most of the Great Barrier reef Queensland (Kott, 1985) (Kott, 2004) ( Australian Mueseum, 2017).
Back to top of page
|
 |
| Figure 9 |
|
|
|
Evolution and Systematics | |
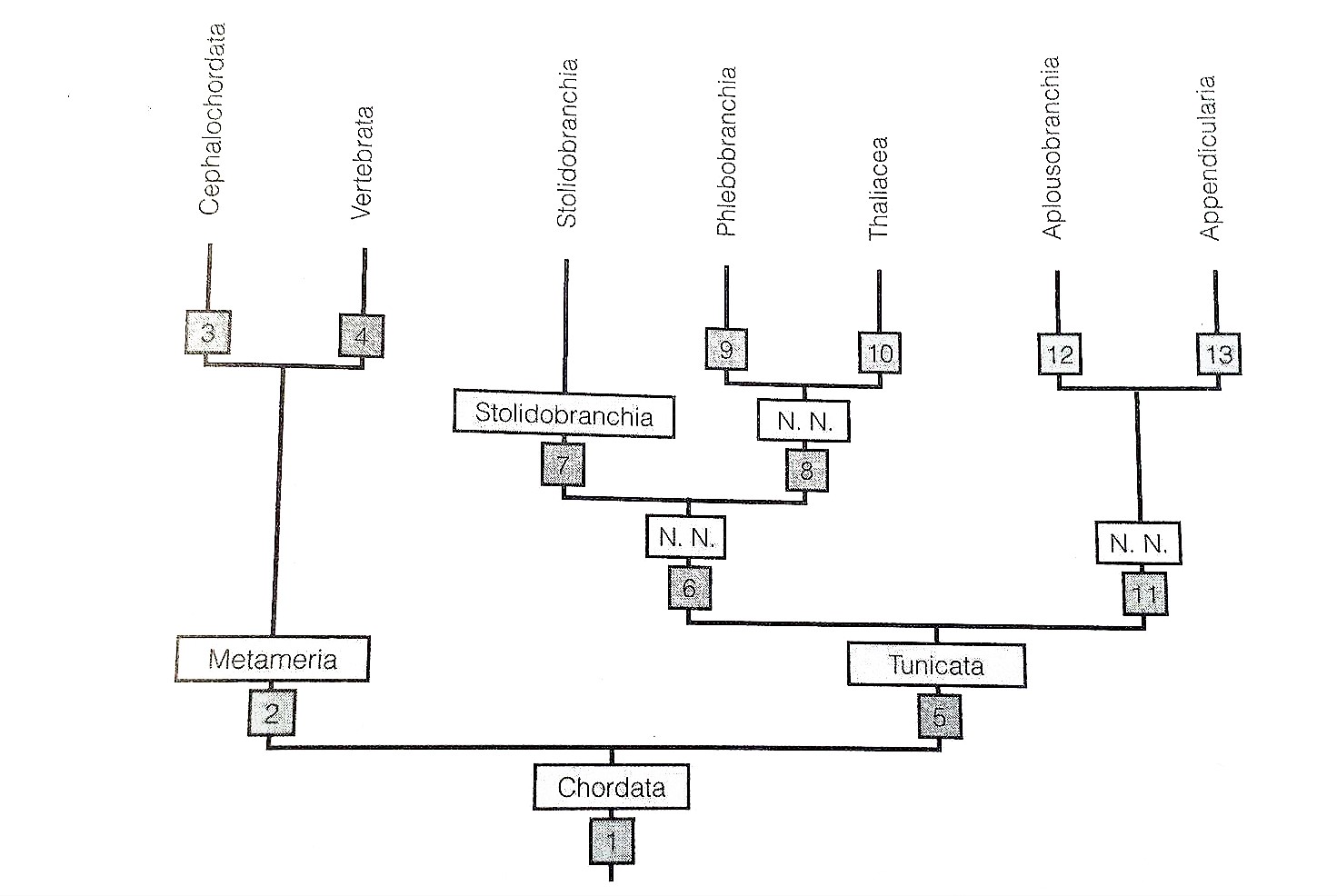
Figure 29: Paraphyletic mapping of Ascidiacea (Tunicata); Thaliacea is sister taxa to the Phlebobranchia (Ascidiacea) and Appendicularia is the sister taxa to Aplousbranchia (Ascidiacea). Phylogeny also shows that Ascidiacea is the sister clade to vertebrates (Ruppert, et al, 2004).
Evolution of Urochodata and Ascidiacea
It is widely accepted that the phylum Urochodata; classes Ascidiacea, Thaliacea and Appendicularia, is the sister taxon to vertebrates, due to anatomical commonalities between the tadpole larva of Urochordata and vertebrate embryos (Schubert, et al, 2006). Both share 5 characteristics of all chordates; a notochord and a hollow dorsal nerve supported by a post-anal tail, an endostyle, and pharyngeal slits (see Larva in Sexual Reproduction) (Ruppert, et al, 2004). Thus Urochodates are considered a key phyla in the evolutionary development of invertebrates (Schubert, et al, 2006).
There is opposing research to say that Ascidiacea is a monophyletic group (figure 30), versus it being a parphyletic group (figure 29) where Thaliacea is sister taxa to the Phlebobranchia (Ascidiacea) and Appendicularia is the sister taxa to Aplousbranchia (Ascidiacea)(Ruppert, et al, 2004).
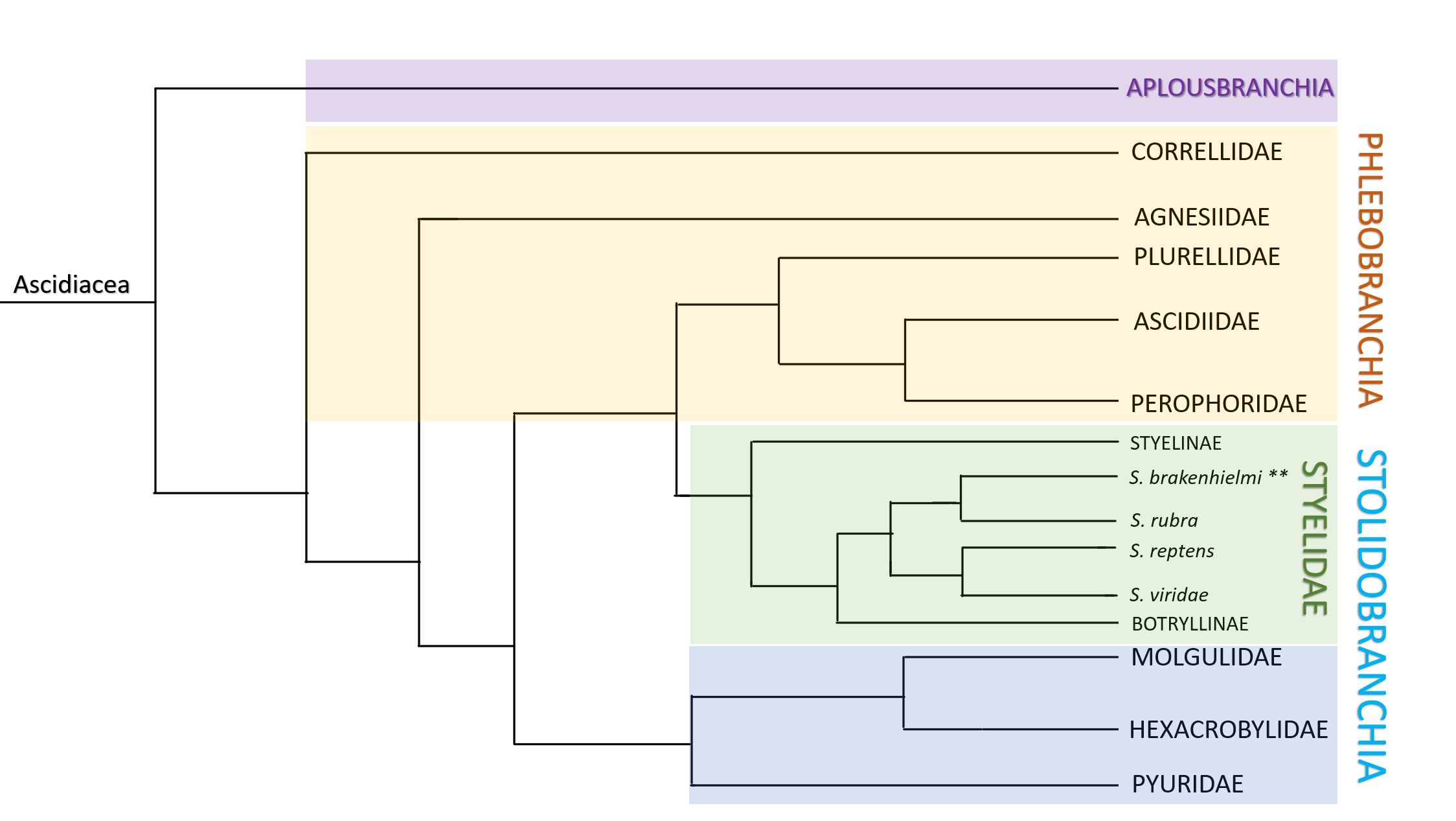
Figure 30: Monophyletic mapping of Ascidiacea. Colours distinguish clades; Aplosbranchia (solitary sp.) = purple, Phlebobranchia (solitary and colonial sp.) = yellow, Stolidobranchia = blue and green. Stolidobranchia contains four discrete families of ascidians; Pyruidae, Molgulidae, Hexacrobylidae (blue) and Styelidae (green). Within Styelidae, there are three subfamilies; Styelinae which are all solitary, and the Botryllinae (includes Botryllus and Botrylloides) and Polyzoinae (includes Symplegma) which are all colonial. Within Polyzoinae is the genus Symplegma, and includes species such as S. brakenhielmi, S. rubra, S. reptens and S. viridae. Note: length of lines does not depict the relative length of time from divergence. Adapted from Kott, 1885 and Pérez-Portela, et al, 2009.
From Solitary to Colonial
Symplegma and Botryllus are apart of the Styelidae ascidians which also includes the solitary clade Styelinae (Kott, 1885). The Styelidae family is separated into colonial and solitary genus (Kott, 1885), as this level is complexity (coloniality) is considered high in evolutionary advancements (Gutierrez and Brown, 2017). Solitary forms are considered more ancestral than coloniality due to their large simple body plan (one heart, one circulatory system, two siphons etc.), whereas zooid integration and regulation in colonial forms involves complex mechanisms to have evolved over time (Gutierrez and Brown, 2017). Recent studies have shown evidence that S. braknehielmi is a transitional species between solitary and colonial forms within the family Styelidae (Gutierrez and Brown, 2017). S. brakenhielmi show ancestral traits of solitary forms - zooids of the colony work independently in the the degradation and generation of other zooids (see Re-absorption and Recycling in Asexual Reproduction). Independence is more obvious within the colony when zooids are at low density, but closer investigation shows that the zooids are still connected by a vascular network of blood vessels and stolons (Gutierrez and Brown, 2017). Although the integration is loose (Gutierrez and Brown, 2017), the zooids work together as a colony to filter the water and to gain and share nutrients (Monniot, et al, 1991). The ability to regenerate zooids through asexual reproduction is greatly assisted by the hemocytes (stem cells in the blood) - the most concentrated types of which are found in S. brakenhielmi, Botryllus and Botrylloides, meaning that these cells are ancestral and arose before the split of semi-colonial (Symplegma) and colonial (Botrillinae)(Gutierrez and Brown, 2017).
Another feature that further defines S. brakenhielmi as being a transitional species is the colony's style of growth and formation - zooids grow asynchronously and their orientation is random (non-polar, unlike Botryllinae) (Kott, 1885). They do not share a common cloacal siphon like Botryllus and Botrylloides (Ruppert, et al, 2004) (Kott, 1885), meaning that their filtration rates and response to stimuli (muscular contractions) of S. brakenhielmi zooids are independent, unlike the species that do share a common cloacal siphon (Ruppert, et al, 2004) (Gutierrez and Brown, 2017). A S. brakenhielmi zooids are very loosely compounded by their common colonial tunic - superficially, their tunic doesn't look like it is shared between zooids, even though it is (stolons are surrounded by tunic which connect the zooids together). This loose integration of the tunic exemplifies further that they evolved from a solitary ascidian related to Styelinae.
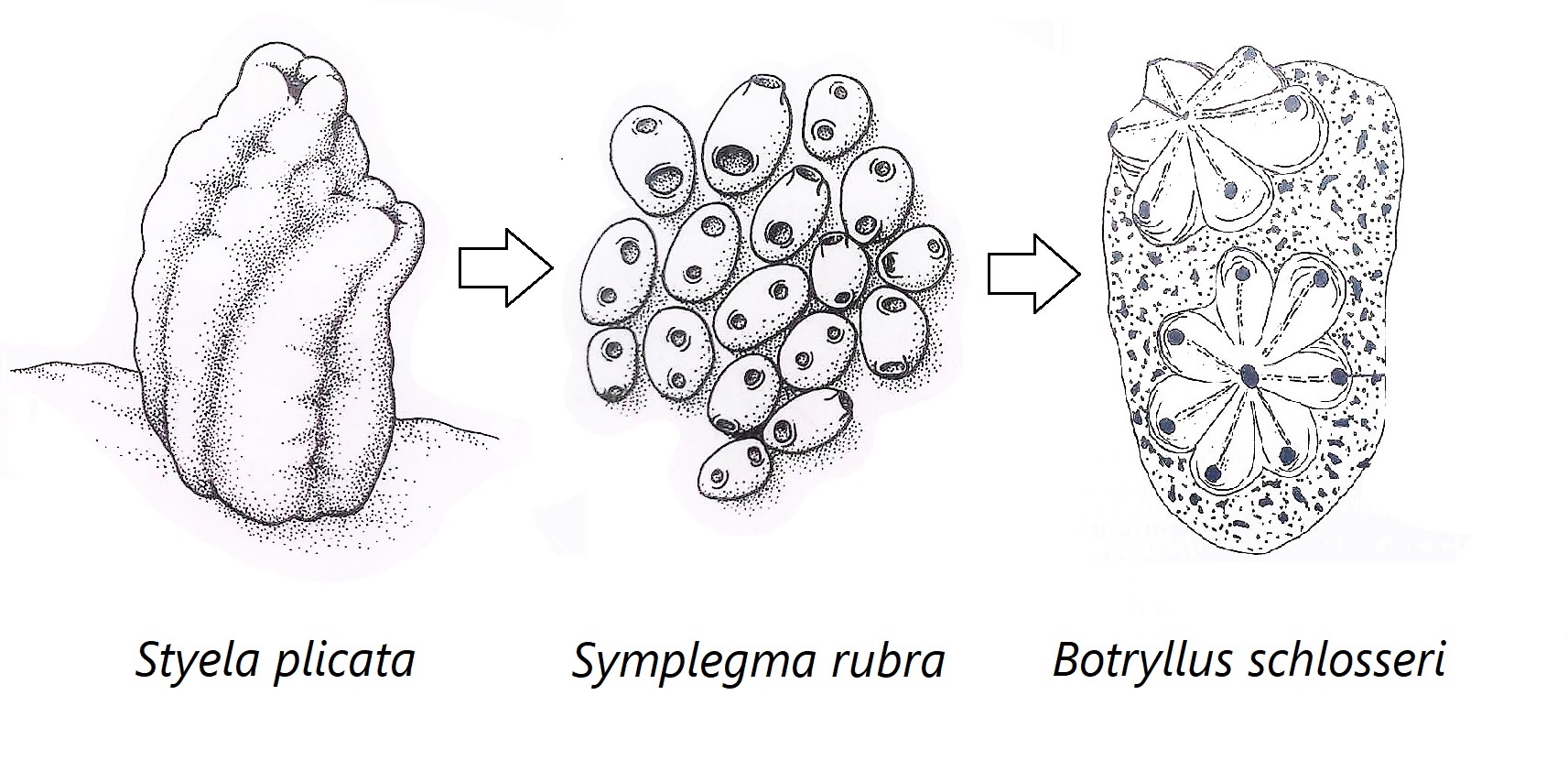 Figure 31: Schematic drawing showing the hypothesised evolutionary changes within Stylidae; includes solitary (Styela plicata), semi-colonial/compound (Symplegma rubra) and compound (Botryllus schlosseri) Stylidae ascidians. S. rubra and S. brakenhielmi show traits of both solitary and compound Stylidae ascidians; independence of zooids in reproductive behaviour, non-polar zooid orientation, vascular network and no common cloacal siphon. Adapted from Ruppert, et al, 2004.
Classification and Systematics
Figure 31: Schematic drawing showing the hypothesised evolutionary changes within Stylidae; includes solitary (Styela plicata), semi-colonial/compound (Symplegma rubra) and compound (Botryllus schlosseri) Stylidae ascidians. S. rubra and S. brakenhielmi show traits of both solitary and compound Stylidae ascidians; independence of zooids in reproductive behaviour, non-polar zooid orientation, vascular network and no common cloacal siphon. Adapted from Ruppert, et al, 2004.
Classification and Systematics
Synonyms of Symplegma brakenhielmi (Michealson, 1904)
- sourced from Kott, 2004
Symplegma oceania (Tokioka, 1961)
Symplegma virde (Michealson, 1904) (Kott, 1952)
Symplegma stuhlmanni (Kott, 1998)
Diandrocarpa brakenhielmi (Michealson, 1904) (Herdman, 1906)
Gynandrocarpa quadricornulis (Sluiter, 1904)
Gynandrocarpa similis (Sluiter, 1904)
Symplegma barani (C. and F. Monniot, 1997) can be distinguished from S. brakenhielmi and it's synonyms by the two sense organs in the papillae of the larva, whereas S. brakenhielmi only has one sense organ (Kott, 2004).
Symplegma rubra (Monniot, 1972) can be distinguished from S. brakenhielmi and it's synonyms by the pigmented ring surrounded the two dorsal siphons and the many testis follicles (up to 15) (Rocha and Costa, 2005).
Back to top of page
|
 |
| Figure 10 |
|
 |
| Figure 11 |
|
 |
| Figure 12 |
|
|
|
Conservation and Threats | |
 S. brakenhielmi is considered to be a world-wide invasive species - disrupting aquaculture systems and competing with stock, and biofouling man-made objects in harbours (Kang, et al, 2011) (Çinar et al, 2006) (see Ecology). The species' ability to grow quickly in warm-water using a variety of budding techniques (see Asexual Reproduction), as well as being able to float on small objects over vast distances has allowed this species to gain a world-wide distribution.
S. brakenhielmi is considered to be a world-wide invasive species - disrupting aquaculture systems and competing with stock, and biofouling man-made objects in harbours (Kang, et al, 2011) (Çinar et al, 2006) (see Ecology). The species' ability to grow quickly in warm-water using a variety of budding techniques (see Asexual Reproduction), as well as being able to float on small objects over vast distances has allowed this species to gain a world-wide distribution.
Instead of being concerned with its protection and conservation, this species and other invasive colonial species (e.g. Botryllus) are purposely being wiped out by farmers in the mussel aquaculture industry (figure 33) (Paetzold, et al, 2012). Using high pressured water currents, the mussels can be cleaned of colonial ascidians and ultimately assisting with the mussel's productivity (Paetzold, et al, 2012). Unfortunately, due to Styelidae ascidian's incredible ability to regenerate, blasting the colony off of the mussel clumps cause fragmentation which increases the species dispersal within the system and worsening the situation (Paetzold, et al, 2012). Methods of removing invasive species like S. brakenhielmi from aquaculture and man-made structures are still being developed.
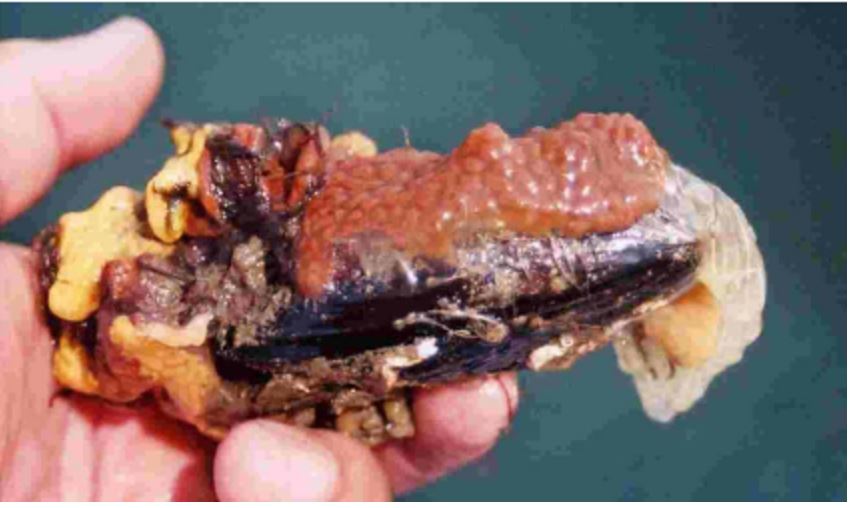 Figure 33: A mussel shell covered in biofouling
Styelid colonial ascidians (Botryllus sp.).
Image source: Minchin, 2007.
Figure 33: A mussel shell covered in biofouling
Styelid colonial ascidians (Botryllus sp.).
Image source: Minchin, 2007.
Back to top of page
|
|
|
References | |
Abdul, J.A.H. and Sivakumar V., 2007. Occurrence and distribution of ascidians in Vizhinjam Bay (south west coast of India). Journal of Experimental Marine Biology and Ecology, 342, 189-190.
Australian Museum, 2017. Atlas of Living Australia: Symplegma brakenhielmi; Viewed 24th of May 2017 <http://bie.ala.org.au/species/urn:lsid:biodiversity.org.au:afd.taxon:96059d3b-9486-4238-98aa-47fc94bc0c8f#tab_recordsView>
Burighel, P., Lane, N.J., Zaniolo, G. and Manni, L., 1998. Neurogenic role of the neural gland in the development of the ascidian, Botryllus schlosseri(Tunicata, Urochordata). Journal of Comparative Neurology, 394, pp.230-241.
Brown, F.D. and Swalla, B.J., 2012. Evolution and development of budding by stem cells: ascidian coloniality as a case study. Developmental biology, 369(2), pp.151-162.
Caicci, F., Zaniolo, G., Burighel, P., Degasperi, V., Gasparini, F. and Manni, L., 2010. Differentiation of papillae and rostral sensory neurons in the larva of the ascidian Botryllus schlosseri (Tunicata). Journal of Comparative Neurology, 518(4), pp.547-566.
Çinar, M.E., Bilecenoglu,
M., Öztürk, B. and Can, A., 2006. New records of alien species on the Levantine
coast of Turkey. Aquatic invasions, 1(2), pp.84-90.
Coles, S.L., DeFelice, R.C., Eldredge, L.G. and Carlton, J.T., 1999. Historical and recent introductions of non-indigenous marine species into Pearl Harbor, Oahu, Hawaiian Islands. Marine Biology, 135(1), pp.147-158.
Corbo, J.C., Di Gregorio, A. and Levine, M., 2001. The ascidian as a model organism in developmental and evolutionary biology. Cell, 106(5), pp.535-538.
Dias, G.M., Duarte, L.F.L. and Solferini, V.N., 2006. Low genetic differentiation between isolated populations of the colonial ascidian Symplegma rubra Monniot, C. 1972. Marine Biology, 148(4), pp.807-815.
Gutierrez, S. and Brown, F.D., 2017. Vascular budding in Symplegma brakenhielmi and the evolution of coloniality in styelid ascidians. Developmental Biology, 423(2), pp.152-169.
Kalk, M., 1970. The organization of a tunicate heart. Tissue and Cell, 2(1), pp.99-118.
Kang, C.K., Choy, E.J., Lee, W.C., Kim, N.J., Park, H.J. and Choi, K.S., 2011. Physiological energetics and gross biochemical composition of the ascidian Styela clava cultured in suspension in a temperate bay of Korea. Aquaculture, 319(1), pp.168-177.
Kott, P. 1985. The Australian Ascidiacea Pt I, Phlebobranchia and Stolidobranchia. Memoirs of the Queensland Museum, 23 1-440.
Kott, P., 2004. Ascidiacea (Tunicata) in Australian waters of the Timor and Arafura seas. Beagle: Records of the Museum and Art Galleries of the Northern Territory, 20, pp 37-81.
Kott, P. 2010 12 30. A review of the Ascidiacea (Tunicata) of Moreton Bay, Queensland. In,Davie, P.J.F. & Phillips, J.A. (Eds), Proceedings of the Thirteenth International Marine Biological Workshop, The Marine Fauna and Flora of Moreton Bay, Queensland. Memoirs of the Queensland Museum - Nature 54(3): 287-297. Brisbane. ISSN 0079-8835.
Koyama, H. and Kusunoki, T., 1993. Organization of the cerebral ganglion of the colonial ascidian Polyandrocarpa misakiensis. Journal of Comparative Neurology, 338(4), pp.549-559.
Kürn, U., Rendulic, S., Tiozzo, S. and Lauzon, R.J., 2011. Asexual propagation and regeneration in colonial ascidians. The Biological Bulletin, 221(1), pp.43-61.
Lambert, C.C. and Lambert, G., 1998. Non-indigenous ascidians in southern California harbors and marinas. Marine Biology, 130(4), pp.675-688.
Lane, N.J., 1972. Neurosecretory cells in the cerebral ganglion of adult tunicates: Fine structure and distribution of phosphatases. Journal of ultrastructure research, 40(5-6), pp.480-497.
Mackie, G.O. and Singla, C.L., 1983. Coordination of compound ascidians by epithelial conduction in the colonial blood vessels. The Biological Bulletin, 165(1), pp.209-220.
Mackie, G.O. and Burighel, P., 2005. The nervous system in adult tunicates: current research directions. Canadian Journal of Zoology, 83(1), pp.151-183.
Maliska, M.E., Pennell, M.W. and Swalla, B.J., 2013. Developmental mode influences diversification in ascidians. Biology letters, 9(3), p.20130068.
Minchin, D., 2007. Rapid coastal survey for targeted alien species associated with floating pontoons in Ireland. Aquatic Invasions, 2(1), pp.63-70.
Monniot, C., Monniot, F. and Laboute, P., 1991. Coral reef ascidians of New Caledonia, 30. IRD Editions.
Murabe, N. and Hoshi, M., 2001. Analysis of the Self-sterility in Halocynthia roretzi. In The Biology of Ascidians, pp. 9-13. Springer Japan.
Olson, R.R. and McPherson, R., 1987. Potential vs. realized larval dispersal: fish predation on larvae of the ascidian Lissoclinum patella (Gottschaldt). Journal of Experimental Marine Biology and Ecology, 110(3), pp.245-256.
Paetzold, S.C., Hill, J. and Davidson, J., 2012. Efficacy of high-pressure seawater spray against colonial tunicate fouling in mussel aquaculture: inter-annual variation. Aquatic Invasions, 7(4), pp.555-566.
Pérez-Portela, R., Bishop, J.D.D., Davis, A.R. and Turon, X., 2009. Phylogeny of the families Pyuridae and Styelidae (Stolidobranchiata, Ascidiacea) inferred from mitochondrial and nuclear DNA sequences. Molecular phylogenetics and evolution, 50(3), pp.560-570.
Pisut, D.P. and Pawlik, J.R., 2002. Anti-predatory chemical defenses of ascidians: secondary metabolites or inorganic acids?. Journal of Experimental Marine Biology and Ecology, 270(2), pp.203-214.
Rocha, R.M.D. and Costa, L.V., 2005. Ascidians (Urochordata: Ascidiacea) from Arraial do Cabo, Rio de Janeiro, Brazil. Iheringia. Série Zoologia, 95(1), pp.57-64.
Rosa, M., Holohan, B.A., Shumway, S.E., Bullard, S.G., Wikfors, G.H., Morton, S. and Getchis, T., 2013. Biofouling ascidians on aquaculture gear as potential vectors of harmful algal introductions. Harmful algae, 23, pp.1-7.
Ruppert, E.E., Barnes, R.D. and Fox, R.S., 2004. Tunicata (Urochordata). Invertebrate zoology: A functional evolutionary appoach. (7th ed.). California: Brooks/Cole.
Schubert, M., Escriva, H., Xavier-Neto, J. and Laudet, V., 2006. Amphioxus and tunicates as evolutionary model systems. Trends in Ecology & Evolution, 21(5), pp.269-277.
Simkanin, C., Dower, J.F., Filip, N., Jamieson, G. and Therriault, T.W., 2013. Biotic resistance to the infiltration of natural benthic habitats: examining the role of predation in the distribution of the invasive ascidian Botrylloides violaceus. Journal of Experimental Marine Biology and Ecology, 439, pp.76-83.
Smithsonian National Museum of Natural History, 2017. Department of Invertebrate Zoology Collection: Symplegma Brakenhielmi, Viewed 24th of May 2017 <http://collections.nmnh.si.edu/search/iz/?q=qn+Symplegma+brakenhielmi>
Stabili, L., Licciano, M., Gravina, M.F. and Giangrande, A., 2016. Filtering activity on a pure culture of Vibrio alginolyticus by the solitary ascidian Styela plicata and the colonial ascidian Polyandrocarpa zorritensis: a potential service to improve microbiological seawater quality economically. Science of The Total Environment, 573, pp.11-18.
Sugimoto, K. and Nakauchi, M., 1974. Budding, sexual reproduction, and degeneration in the colonial ascidian, Symplegma reptans. The Biological Bulletin, 147(1), pp.213-226.
Swalla, B.J., 2006. Building divergent body plans with similar genetic pathways. Heredity, 97(3), pp.235-243.
Youssef, D.T., Mohamed, G.A., Shaala, L.A., Badr, J.M., Bamanie, F.H. and Ibrahim, S.R., 2015. New purine alkaloids from the Red Sea marine tunicate Symplegma rubra. Phytochemistry Letters, 13, pp.212-217.
Back to top of page
|
|
|
|
|